Predicting Corrosion Rates
Armed with the Tafel equation and Tafel plots, it is now possible to predict whether a particular setup will result in corrosion and if so how fast the corrosion will be.
In order for corrosion to occur, there must be a suitable anodic reaction and an appropriate cathodic reaction. This is manifested as an intersection of a cathodic branch and an anodic branch on a Tafel plot. The point of intersection gives the corrosion potential and the corrosion current (or, more accurately the log of the corrosion current density).
The rate of corrosion is governed by all the factors discussed previously. When all the effects are taken into account, Tafel plots get quite complicated and some interesting effects occur:
Faraday’s law allows the current density to be expressed as the mass of material lost per unit time.
The calculation involves a few simple steps. For a corrosion reaction:
- The current is converted into a rate of electron consumption using the electronic charge constant.
- The number of electrons is divided by the stoichiometric number of electrons in the corrosion reaction, giving the number of metal atoms lost per unit time.
- This answer is then divided by Avogadro’s number to give the number of moles of metal atoms lost per unit time.
- The number of moles is then converted to mass lost per unit time, using the molar mass.
- The mass is then converted to a volume using the density.
- The volume is then converted to a thickness lost per unit time by dividing by the area that the current passes over. If a current density was given, this step has already been done.
Overall, the thickness of metal lost per unit time is given by the formula:
$$t = {{i{m_{\rm{M}}}} \over {\rho ez{N_{\rm{A}}}}}$$
where t = thickness (m), i = current density (A m-2), mM = molar mass (kg mol-1), e = electronic charge (C), z = stoichiometric number of electrons in oxidation reaction, NA is Avogadro’s number.
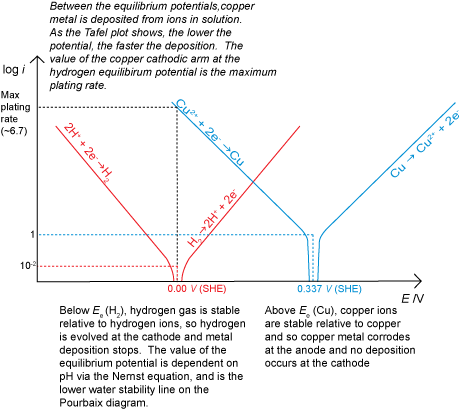
It is also possible to have a situation where corrosion does not occur for thermodynamic reasons, for example if there was a driving force for the reverse of the corrosion reaction to occur due to an applied potential. This would result in deposition (electroplating) if there were metal ions in solution available to be reduced. If deposition is being carried out commercially, for example to electroplate silver onto stainless steel cutlery, the rate must be maximised to make production as cost effective as possible. However, care must be taken to avoid the hydrogen evolution reaction starting at the cathode in addition to the metal ion deposition.
We can now draw Tafel plots and use them to determine corrosion current densities and corrosion rates. Below is an interactive graph that allows the corrosion rates of several metals to be investigated. Notice how, in this idealised situation, i.e. with no diffusion limits, the corrosion rate in aerated water can be extremely high. This shows how important a consideration the diffusion layer is.

