Crystal systems
The rotational symmetry of a crystal places constraints on the shape of the conventional unit cell we choose to describe the structure. On this basis we divide all structures into one of 7 crystal systems. For example, for crystals with 4 fold symmetry it will always be possible to choose a unit cell that has a square base with a = b and γ = 90°:
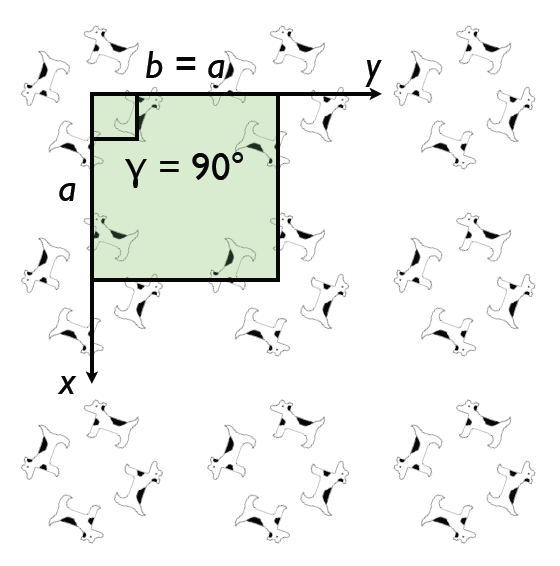
There are 14 unique combinations of the 7 crystal systems with the possible types of primitive and non-primitive lattices. These are referred to as the 14 Bravais lattices.
Crystal systems, lattices and symmetry elements
Crystal System |
Defining Symmetry |
Unit Cell Geometry |
|
Triclinic |
Translational Only |
a≠b≠c; α≠β≠γ |
|
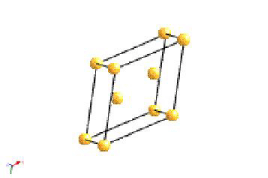
Monoclinic |
A diad axis |
a≠b≠c; α=γ=90°; β>90° |
|
Orthorhombic |
3 diads |
a≠b≠c; α=β=γ=90° |
|
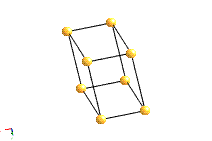
Trigonal For more information click here |
1 triad |
a=b≠c; α=β=90°; |
|
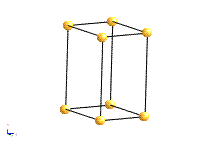
Hexagonal |
1 hexad (parallel to [001]) |
a=b≠c; α=β=90°; |
|
Tetragonal |
One tetrad |
a=b≠c; α=β=γ=90° |
|
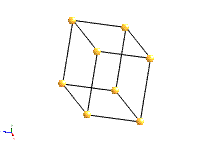
Cubic |
4 triads |
a=b=c; α=β=γ=90° |
|
Bravais Lattice Structures
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|