Slip systems in sodium chloride
Sodium chloride is cubic F with Cl atoms at 0,0,0 and Na atoms at 0,0,½. A unit cell of the material is shown below.
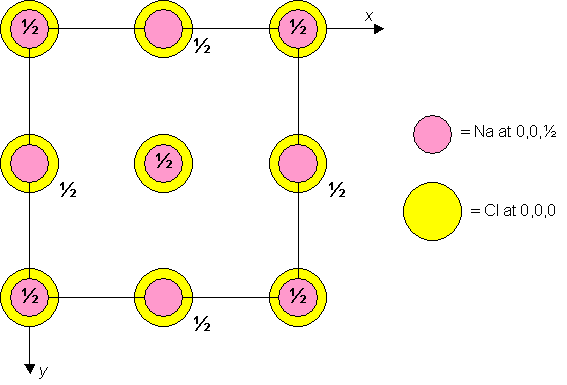
Unit cell plan for sodium chloride.
Dislocation glide (or slip) generally occurs in the direction of the shortest lattice vector. In the sodium chloride structure, therefore, the shortest lattice vectors connect lattice points such as 0,0,0 and 0,0,½, across half of the face diagonal. Try and identify these vectors on the 3D model of the unit cell.
Virtual reality model of a sodium chloride unit cell
Using the Miller index notation, these vectors are in <1 bar1 0> directions. The planes on which slip occurs are slip planes - in sodium chloride, these are {110} planes. Therefore we say that sodium chloride slips on {110}<1 bar1 0> slip systems.
The etch pits in the deformed surface appear schematically as follows:
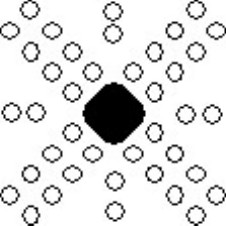
Schematic showing alignment of etch pits around deformed region.
The diagrams have been drawn with the sides of the crystal horizontal and vertical, and it is assumed that the surface is a (001) plane. Etch pits arise when a dislocation line intersects with the surface, and since the dislocation line must lie in the slip plane, the orientation of the etch pits can be matched to the slip planes and the slip directions, as shown in the diagram below. A row of etch pits in the rosette corresponds to the intersection of slip planes with a specific orientation intersecting with the surface.
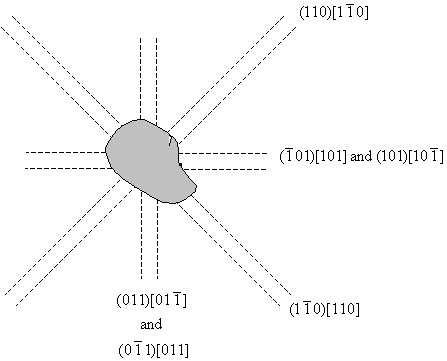
Miller indices for etch pit orientations.

