The piezoelectric dipole moment
When a piezoelectric is placed under a mechanical stress, the atomic structure of the crystal changes, such that ions in the structure separate, and a dipole moment is formed. For a net polarisation to develop, the dipole formed must not be cancelled out by other dipoles in the unit cell. To do this, the piezoelectric atomic structure must be non-centrosymmetric, i.e. there must be no centre of symmetry. Materials which are centrosymmetric, when placed under stress, experience symmetrical movement of ions, meaning there isn't a net polarisation. This can be seen in the following picture, in which there is an atom in a tetrahedral interstice. This material is ZnS, sphalerite, a Zn f.c.c. structure, which has an S atom in half of the tetrahedral interstices.
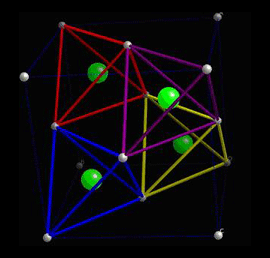
Locally, in each interstice, there is no centre of symmetry, so when a stress is applied, the motion of the central atom results in a dipole moment. Consider a single tetrahedra:
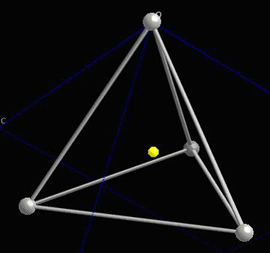
When the central atom moves, a dipole moment forms:
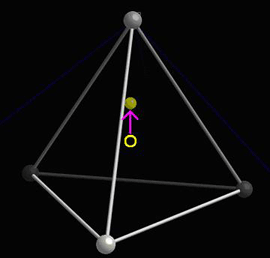
See the section on The dipole moment in the Ferroelectric Materials TLP for more information on the associated mathematics.

