Crystallinity
Crystallinity defines the degree of long-range order in a material, and strongly affects its properties. The more crystalline a polymer, the more regularly aligned its chains. Increasing the degree of crystallinity increases hardness and density. This is illustrated in poly(ethene).
HDPE (high density poly(ethene)) is composed of linear chains with little branching. Molecules pack closely together, leading to a high degree of order. This makes it stiff and dense, and it is used for milk bottles and drainpipes.
The numerous short branches in LDPE (low density poly(ethene)) interfere with the close packing of molecules, so they cannot form an ordered structure. The lower density and stiffness make it suitable for use in films such as plastic carrier bags and food wrapping.
Often, polymers are semi-crystalline, existing somewhere on a scale between amorphous and crystalline. This usually consists of small crystalline regions (crystallites) surrounded by regions of amorphous polymer.
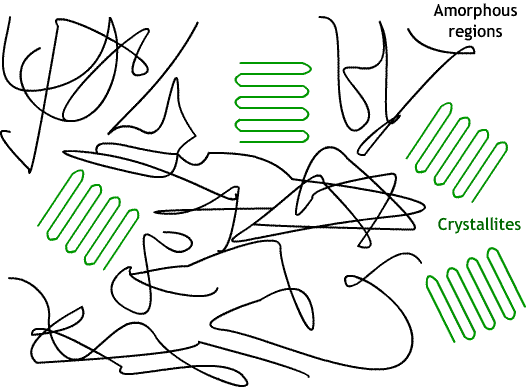
Factors favouring crystallinity
In general, factors causing polymers to be more ordered and regular tend to lead to a higher degree of crystallinity.
- Fewer short branches – allowing molecules to pack closely together
- Higher degree of stereoregularity - syndiotactic and isotactic polymers are more ordered than atactic polymers.
- More regular copolymer configuration – having the same effect as stereoregularity
This topic is covered in the Crystallinity in Polymers TLP.

