Shape, size & structure I
Configuration and conformation
Configuration is a property which encompasses:
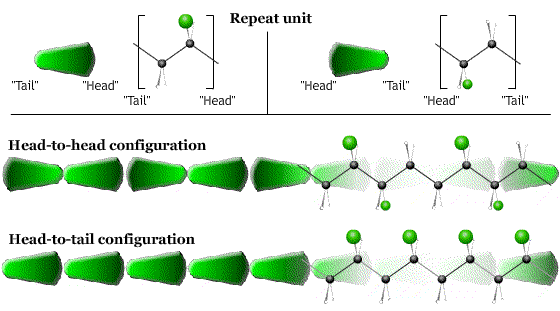
- The direction (head-to-tail, head-to-head …) in which the monomers are linked together.
- The order (ABABAB…) in which monomers are joined together (in polymers with more than one type of monomer).
The configuration is fixed during synthesis.
After synthesis, the molecule can change its shape by rotations about the single C-C bonds in the backbone. The particular arrangement of the chain due to these rotations is called the conformation.
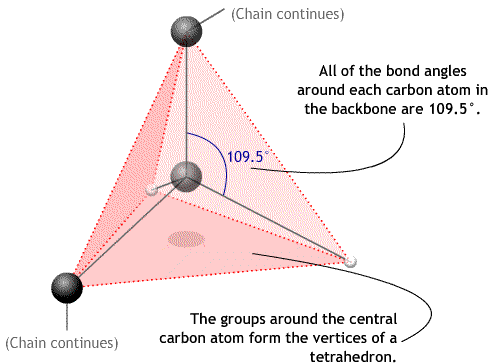
The angles between bonds around a carbon atom in the backbone are approximately 109.5°. This is the bond angle and is contant.
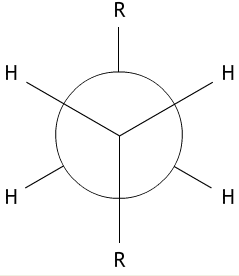
We can illustrate conformation using a Newman projection. This is a diagram which shows the arrangement of the side groups, looking down the C-C bond (represented by a circle) from one carbon atom to the other. Newman projections clearly show the torsion angle – the angle through which one carbon atom is rotated relative to the next.
There is a potential energy that comes from the interaction of side groups on adjacent carbon atoms in the chain. This energy varies as a function of the torsion angle, as side groups move relative to each other. The torsion angles with the lowest energy are more stable. These are called trans, gauche + and gauche -. The conformations that a chain will preferentially adopt are sequences of these three stable angles.
The following application is an introduction to Newman projections using the example of butane. It also illustrates the change in potential energy with torsion angle for butane.
The idea of Newman projections for butane can be extended to longer chains. In this case the CH3 groups on the diagram would be replaced by the symbol R, which stands for any group of atoms. This is shown below.
Using the following application you can build a chain of poly(ethene) with your own conformation, choosing In order to change between favourable torsion angles, the molecule must pass through those with higher energies. This represents a potential energy barrier to conformation changes.
The more energy available to a molecule, the more readily it may change its conformation, and its stiffness decreases. However, the more flexible the molecule, the more likely it is to adopt a random conformation, so the stiffness of the polymer material itself may actually increase with temperature.
Much more detail on conformation & stiffness in the context of rubber can be found in the stiffness of rubber TLP.
Below a certain temperature, the glass transition temperature Tg, a molecule’s internal energy is low enough that changes in shape of a molecule cannot be effected by conformation changes, but must instead be accommodated by stretching intermolecular bonds. The properties of a polymer change drastically below its glass transition temperature. This area is explored in the glass transition in polymers TLP.

