Shape, size & structure II
Individual chains can be linked together to produce a branched structure or a network.
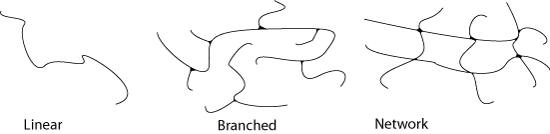
Branching
Branches normally form because of side reactions that happen during the synthesis of a polymer. The degree of branching can be controlled to obtain different properties, for example by adding a second type of monomer chosen to promote branch formation.
Cross-links
Whereas branching involves joining the head of one chain to a point in the middle of another, cross-linking joins two chains together at some point along their length. Cross-linking in a polymer forms a network structure. A network is in fact one giant molecule because the cross-links are primary chemical bonds.

Without cross-links, van der Waals forces hold adjacent molecules together. These are weak attractions, caused by the interaction of electrons. They break easily on heating, allowing the molecules to slide past each other. Substances made from un-cross-linked polymers are therefore able to melt, and are called thermoplastics.
Cross-links are primary chemical bonds, which require much more energy to break than van der Waals forces. When a polymer of this type is heated, the covalent cross-links prevent individual molecules from sliding past one another, so melting does not occur. This type of polymer is called a thermoset. At a sufficiently high temperature, the covalent bonds both in the cross-links and within the molecules are broken, and the thermoset decomposes.
A polymer with only a small degree of cross-linking is an elastomer. The cross-links are infrequent enough to allow significant conformation changes in the chains between cross-links, while still preventing individual molecules from flowing past each other. Elastomers can accommodate a large amount of recoverable deformation, behaviour typical of rubber. Much more information about the behaviour of elastomers is available in the Stiffness of Rubber TLP.
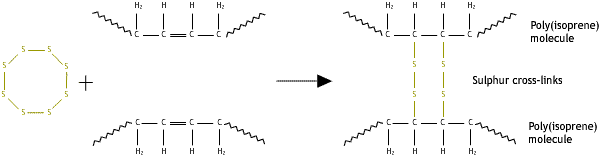
Cross-links may be added after synthesis by a separate process. One such process is vulcanisation, where cross-links are added to rubber by heating it with sulphur.