Demonstration of phase separation
The transition from a single phase to two phases (and vice-versa) can be easily demonstrated using a mixture of two suitable liquids.
A mixture of cyclohexane and aniline can exist as two separate phases or as a single phase, depending on the temperature. The thermodynamic transition between these two states can be understood by considering the balance between entropy and enthalpy (see Thermodynamics section in this TLP).
Aniline and cyclohexane are immiscible over a wide range of compositions below about 35 ºC. In this immiscible region there exists an aniline-rich and a cyclohexane-rich phase, separated by a boundary, seen as a meniscus. When this mixture is heated, the volume of one phase increases at the expense of the other. This can be seen as movement of the meniscus, provided the heating is slow enough. At the transition temperature for the particular composition, there will no longer be two discernable phases.
A significant point occurs when the distinction between the two coexisting phases reduces to zero. Here the domains present in the mixture can switch easily between aniline-rich and cyclohexane-rich. The composition variance is on such a scale as to interfere with light passing through it. Light will scatter in proportion to the squared difference in n, the index of refraction, of the two phases. Therefore light scatters more as the number of domain interfaces increases. Hence light is scattered strongly by the mixture across a small temperature range around the transition temperature.
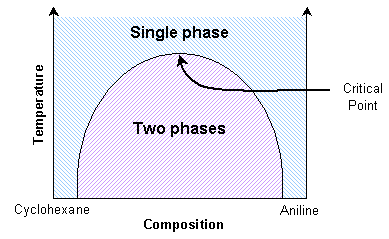
At the critical point, the scattering is so intense that the system becomes opaque. This phenomenon is called critical opalescence. The domains demonstrate some interesting properties, such as fractal shapes, and there is a peak in the heat capacity. This critical transition temperature is a maximum with respect to the composition. Thus it can be determined by interpolating transition temperatures from known compositions.
Demonstration
A mixture of equal quantities of cyclohexane and aniline contained in a sealed vial is heated to approximately 35 ºC (i.e. just above the critical temperature), using a water bath (or water from a hot tap). The mixture is allowed to cool, and a laser is pointed at the vial so that it shines onto a screen opposite, as shown in the diagram below.
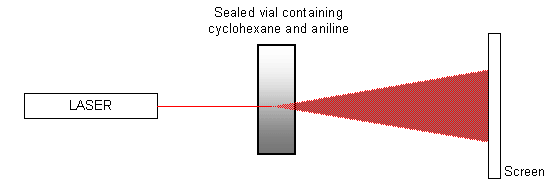
When the critical temperature is reached and the mixture goes from a single phase to two phases, the spot of light on the screen is disrupted as the phases separate. The spot ‘flickers' and then becomes totally diffuse. It will eventually form a single spot again once the transition is completed and the two chemicals have completely separated. The pattern of events can also be seen in reverse as the mixture is heated.
Video showing the laser light (as seen on the screen) flickering and spreading out to become
completely opaque as the mixture cools through the transition temperature
The video has been speeded up by a factor of about 10.
Video showing the vial, which has been filmed perpendicular to the direction of the laser light (left to right)
Initially, the laser light is seen as a single beam passing through the mixture, but as the transition point is reached, the beam spreads out. The single beam eventually reforms, once the transition is complete. This video has been speeded up by a factor of about 200.

