Steady State Solute Profile ahead of an Advancing Solidification Front
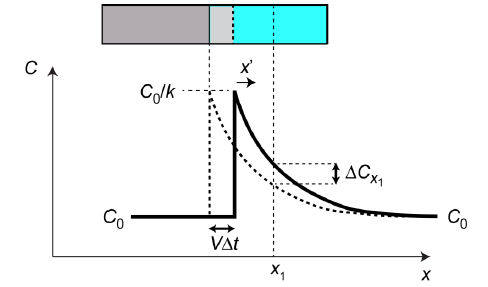
Fig.1 Changing profile during incremental advance of the interface, in a fixed frame (x).
During a time interval Δt, the front advances by VΔt, where V is its velocity. There is no change in the coordinates of physical matter in the fixed frame (neglecting the effect of the volume change on freezing). Any particular slice of liquid, eg at x = x1, will therefore change in composition only as a result of diffusion (governed by the diffusion equation).
$${{\partial C} \over {\partial t}} = D{{{\partial ^2}C} \over {\partial {x^2}}}$$
so that, in time Δt,
$$\Delta {C_{{x_1}}} = \Delta tD{\left. {{{{\partial ^2}C} \over {\partial {x^2}}}} \right|_{x = {x_1}}}$$
Since the profile is in steady state, when referred to the moving frame x’ (= x – Vt), the composition must be constant for all values of x’. This requires
$${\left. {{{\partial C} \over {\partial x}}} \right|_{x = {x_1}}} = {{\Delta {C_{{x_1}}}} \over {\left( { - V\Delta t} \right)}}$$ $$ ∴ - V\Delta t{\left. {{{\partial C} \over {\partial x}}} \right|_{x = {x_1}}} = \Delta tD{\left. {{{{\partial ^2}C} \over {\partial {x^2}}}} \right|_{x = {x_1}}}$$ $${\rm{i}}{\rm{.e}}{\rm{. }}\;D{{{\partial ^2}C} \over {\partial {x^2}}} + V\left( {{{\partial C} \over {\partial x}}} \right) = 0\;\;\;{\rm{for\;\;all\;\;x}}$$
Since x’ is given by (x – Vt), this equation must also hold in the x’ frame.
A solution is now required to this equation that satisfies the boundary conditions. Integrating
$$D\left( {{{\partial C} \over {\partial {x{^′}}}}} \right) = - VC +{\rm{const}}$$
This constant is found by equating the rate of rejection of solute across the interface to the rate at which it is diffusing down the concentration gradient at x’ = 0.
$$D{\left. {{{\partial C} \over {\partial {x^′}}}} \right|_{x = 0}} = - \left( {{{{C_0}} \over k} - {C_0}} \right)V$$ $$ ∴ - \left( {{{{C_0}} \over k} - {C_0}} \right)V + V\left( {{{{C_0}} \over k}} \right) = {\rm{const}}$$
∴ const = V C0
The resultant equation is then integrated again
$$D\left( {{{\partial C} \over {\partial {x^′}}}} \right) = - V(C - {C_0})$$ $${{\partial C} \over {(C - {C_0})}} = - {V \over D}\partial {x^′}$$ $$\ln (C - {C_0}) = - \left( {{V \over D}} \right){x^′} + {\rm{const}}$$
This constant is found by setting C equal to C0/k at x’ = 0
$${\rm{const = ln}}\left( {{{{C_0}} \over k} - {C_0}} \right)$$
The solution can thus be written:
$$\ln (C - {C_0}) - {\rm{ln}}\left( {{{{C_0}} \over k} - {C_0}} \right) = - \left( {{V \over D}} \right){x^′}$$ $${{(C - {C_0})} \over {\left( {{{{C_0}} \over k} - {C_0}} \right)}} = \exp \left( { - \left( {{V \over D}} \right){x^′}} \right)$$ $$C = {C_0}\left[ {1 + \left( {{{1 - k} \over k}} \right)\exp \left( {{{ - {x^′}} \over {D/V}}} \right)} \right]$$
Incidentally, it follows that the concentration gradient at any point ahead of the interface is given by
$${{\partial C} \over {\partial {x^′}}} = {C_0} {\left( {{{1 - k} \over k}} \right)\left( {{{ - 1} \over {D/V}}} \right)\exp \left( {{{ - {x^′}} \over {D/V}}} \right)}$$
and, in particular, this gradient is given by the following expression at the interface
$${\left. {{{\partial C} \over {\partial {x^′}}}} \right|_{{x^′} = 0}} = - {{\left( {{{{C_0}} \over k} - {C_0}} \right)} \over {D/V}}$$
The numerator in this expression is the composition difference across the interface, so the denominator must be the distance indicated in Fig.2. It’s often termed the solute boundary layer (δ) and it can be defined either via the above equation or as the characteristic distance for the exponential decay (see Fig.2). The above equation represents a simple and useful way of expressing the concentration gradient at the interface.
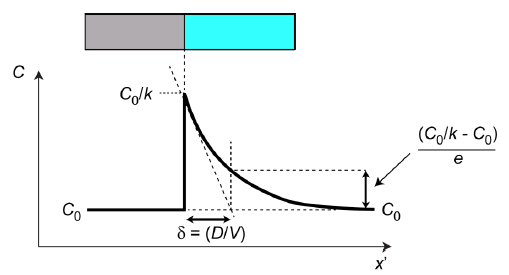
Fig.2 Steady state profile, showing how the solute boundary layer (δ) is defined.

