Entropy derivation
It is possible to treat quantitatively the entropy change on extending a polymer chain:
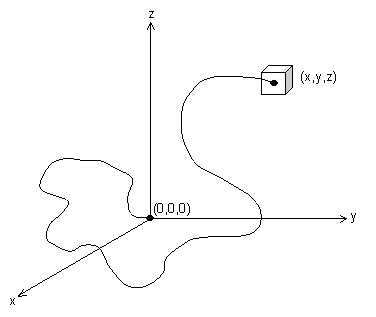
If one end of the chain is at (0,0,0), then the probability of the other end being at point (x,y,z) is:
\[p\left( {x,y,z} \right).dx.dy.dz = {\left( {\frac{b}{{\sqrt \pi }}} \right)^3}\exp \left[ { - {b^2}\left( {{x^2} + {y^2} + {z^2}} \right)} \right].dx.dy.dz\]
where
\[b = \sqrt {\frac{3}{{2n{a^2}}}} \]
a = bond length
n = number of links
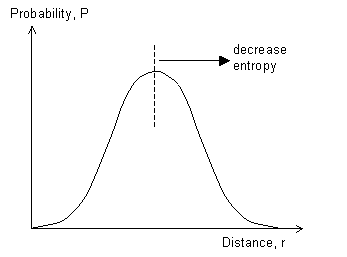
If we stretch the chain, so that the end is at a new location (x',y',z') such that (x'2 + y'2 + z'2) > (x2 + y2 + z2), then p(x,y,z) will decrease, leading to a decrease in entropy. The entropy is given by
S = k lnΩ
where Ω = total number of possible conformations leading to the same end position. Now
Ω = p(x,y,z,)
On stretching a chain, so that the initial end point (x,y,z) changes to (x',y',z') where
x' = λxx
y' = λyy
z' = λzz
the associated change in entropy is given by
\[\Delta S = k\ln \left( {\frac{{\Omega '}}{\Omega }} \right) = k\ln \left( {\frac{{p'}}{p}} \right)\]
ΔS = −kb2 [((x')2 − x2) + ((y')2 − y2) + ((z')2 −z2)]
ΔS = −kb2 [(λx2 − 1) x2 + (λy2− 1)y2 + (λz2 − 1)z2]
In the unstressed state, with overall length r, we expect no preferential direction, so:
\[\left\langle {{x^2}} \right\rangle = \left\langle {{y^2}} \right\rangle = \left\langle {{x^2}} \right\rangle = \frac{{\left\langle {{r^2}} \right\rangle }}{3}\]
So, on average:
\[\Delta S = - k\left( {\frac{3}{{2n{a^2}}}} \right).\frac{{\left\langle {{r^2}} \right\rangle }}{3}.\left( {\lambda _x^2 + \lambda _y^2 + \lambda _z^2 - 3} \right)\]
From random walk theory,
〈 r2 〉 = na2
The entropy of a single chain segment can be multiplied by N (the no of chain segments) to give the total entropy change:
\[\Delta {S_{{\rm{tot}}}} = - \frac{1}{2}Nk\left( {\lambda _x^2 + \lambda _y^2 + \lambda _z^2 - 3} \right)\]

