The Lennard-Jones potential
The expansion of materials as their temperature is increased can be explained on a molecular level by the increased average spacing between atoms or molecules as they vibrate with greater amplitude at higher temperatures. This can be shown from the graph of potential energy against bond length, the Lennard-Jones potential.
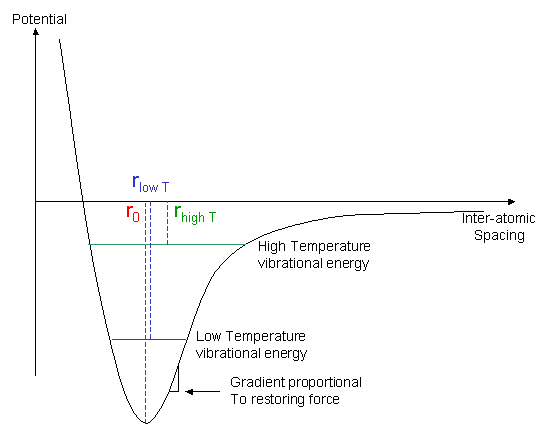
In the graph, r0 is the equilibrium bond length, i.e. the bond length when there is no atomic vibration, and r is the average bond length at an elevated temperature.
As the temperature is increased, the thermal energy available to the material increases, so the average vibrational energy of the bonds increases. It can be seen from the asymmetric form of the graph that this increase in energy will cause an increase in the average bond length. Under normal circumstances, this will cause the material to expand.
Furthermore, the plot illustrates why stiffness normally falls with increasing temperature. When a bond is stretched by an external load, the restoring force (tending to return the average bond length to its equilibrium value) is proportional to the gradient of this energy-separation curve. At high temperatures (vibration amplitudes), this restoring force is, on average, lower than at low temperatures.

