Superelasticity and Shape Memory Alloys (all content)
Note: DoITPoMS Teaching and Learning Packages are intended to be used interactively at a computer! This print-friendly version of the TLP is provided for convenience, but does not display all the content of the TLP. For example, any video clips and answers to questions are missing. The formatting (page breaks, etc) of the printed version is unpredictable and highly dependent on your browser.
Contents
Main pages
Additional pages
Aims
On completion of this TLP you should understand:
- how Deformation Twinning and Martensitic Transformations can generate a shape change
- the phenomenon of Superelastic Deformation, which is a reversible Shape Change effected by Martensitic Transformations
- the role of temperature, which affects the driving force for a Martensitic Transformation, and can also influence the ease of slip (dislocation motion)
- the Shape Memory Effect, in which temperature changes are used (in alloys with certain characteristics) to promote reversible Martensitic Transformations and hence to control component shape
Before you start
It might be useful to understand how localised strain varies within a spring subjected to axial extension, which is covered in the Bending and Torsion of Beams TLP. However, the issue is relevant only to a small part of this TLP.
It would be helpful to have a clear understanding of the basic concepts of thermodynamics. These can be found in the Phase Diagrams TLP and the Ellingham Diagrams TLP.
Introduction
Certain metallic alloys (and also some polymeric and ceramic materials) exhibit unusual behaviour when subjected to mechanical load and/or temperature change. This is due to shape changes being generated by Martensitic Phase Transformations, rather than by conventional elastic (bond stretching) or plastic dislocation glide (Introduction to dislocations TLP) deformation. Something very similar happens during Deformation Twinning. However, unlike the case of twinning, these phase transformations are reversible, at least under certain conditions, and hence the associated shape change can also be reversed. This can lead to interesting and useful effects, such as a capacity to cycle a component between two different macroscopic shapes by cycling the temperature. These alloys are commonly known as Shape Memory Alloys (SMAs).
The most common of these alloys is an equi-atomic alloy of Ni and Ti, known as ‘Nitinol’, although both Ni-rich and Ti-rich alloys are also used. Other examples include Cu-Zn or ternary alloys like Ni-Cu-Ti or Ni-Hf-Ti. They have been used for a variety of applications, including pipe couplings, earthquake dampeners, eyeglass frames, orthodontic wires, mobile phone antennas, micro-actuators, and a variety of biomedical devices.
Martensitic Phase Transformations - A Simple Example
Martensitic transformations are diffusionless shear transformations. The transformation is most commonly driven by mechanical deformation or by a change in temperature. A martensitic transformation is an example of a displacive transition, in which there is cooperative motion of a relatively large number of atoms, each being displaced by only a small distance (a fraction of an interatomic spacing) relative to its neighbours. Click here to see the difference between displacive and diffusional transformations.
A simple example of a martensitic phase transformation is provided by the transition from cubic close-packed (ccp) to hexagonal close-packed (hcp). This occurs by the systematic sliding of close-packed planes, ie {111} planes in ccp, over one another. As it happens, the same kind of sliding (in the same direction and by the same distance) can also lead to formation of a twin (ie the same ccp crystal structure, but in a different orientation). A twin is formed when every (111) plane undergoes this displacement, relative to the plane below it, whereas the hcp structure is created when every second (111) plane does this.
Martensitic Phase Transformations - Basic Thermodynamics
Some simple features of the thermodynamics relevant to phase transformations are given here - see Ellingham Diagram TLP. The quantity of primary concern here is the Gibbs free energy, G.
The animation below shows the stability of two phases as a function of temperature. For superelastic and shape memory alloys, the two phases are normally termed “austenite” (stable at higher temperatures) and “martensitic” phase (stable at lower temperatures). Sometimes, the austenitic phase is termed the “parent” phase.
It is important to be clear that this terminology is generic – i.e., these phases are not any specific ones, but refer to a type of phase. In particular, it is important to avoid any confusion with the austenite and martensite phases that form in steels. As it happens, while the austenite-to-martensite transformation that occurs in the Fe-C system obviously is a martensitic transformation, it is crystallographically complex and exhibits certain rather special characteristics. Moreover, for reasons that need not be detailed here, superelasticity and shape memory behaviour are NOT normally exhibited by steels.
Footnote: Although commonly presented in this form, it's not strictly correct for the free energy to be shown as decreasing with increasing temperature. It actually increases, despite the -TS term, because the enthalpy increases approximately linearly with temperature (with the proportionality constant being the specific heat of the material). However, when comparing two phases (with different entropies, but similar specific heats) in this way, the changes in enthalpy are often neglected. Both plots then decrease with increasing T (due to the -TS term), with the decrease being at a higher rate for the (more disordered) phase with higher entropy. (This is why highly disordered phases, such as liquids and gases, tend to be stable at higher temperatures.)
Martensitic Phase Transformations - Hysteresis Characteristics
Ideally, leaving aside the possibility of phases forming with different compositions, the most stable phase (ie that with the lowest free energy) should be present at any given temperature. However, in reality a phase may persist beyond the temperature range in which it is thermodynamically stable. This is because a driving force is often required in order to form the new phase within the existing phase. For example, a common cause of this is a nucleation barrier, which is associated with the large interfacial energy contribution, per unit volume, for small transformed volumes.
A further potential source of such behaviour, which applies only when both phases are solid, and is particularly important for shear transformations, is the stored elastic strain energy that arises when a region undergoes a shape change as it transforms. Unlike the case of a nucleation barrier, the energy penalty associated with this elastic strain continues to rise as larger volumes of material transform. The upshot of this is that pronounced hysteresis is commonly observed in the phase transformations that occur during thermal cycling of SMAs.
- The austenitic phase start temperature, As, which is the temperature where the martensitic phase begins to transform into the parent (austenitic) phase.
- The austenitic phase end temperature, Af, which is the temperature where the martensitic phase has completely transformed in the austenitic phase.
- The martensite start temperature, Ms, which is the temperature where the austenitic phase starts to transform into the martensite phase.
- The martensite end temperature, Mf, which is the temperature where the austenitic phase has completely transformed into the martensite phase.
These temperatures are not very well-defined, since they are dependent on experimental conditions (for example heating and cooling rates).
Superelasticity - Strain Accommodation by Martensite Formation
Superelasticity (SE), sometimes termed “pseudo-elasticity” or “pseudo-plasticity”, occurs without any change in temperature. SE takes place at temperatures above As - although usually only slightly above - where the austenitic phase is the more stable of the two thermodynamically, although not by very much. When a mechanical strain is imposed, this can stimulate the transformation of austenite to martensite, sometimes termed “stress-induced martensite”. The associated shear of local regions accommodates the imposed macroscopic shape change, while the lower strain energy component ensures that the overall free energy is now lower than it would be if the austenitic phase were still predominant.
Relatively large strains (up to about 8%) can be accommodated in this way. As the strain is increased, the proportion of the specimen that has transformed to martensite progressively rises. This occurs without much increase needed in the applied stress, giving rise to a characteristic “superelastic plateau”.
These strains are much higher than would normally be possible during conventional elastic deformation (up to ~0.5% for most metals). Nevertheless, they are recoverable when the applied load is removed. When this is done, the material reverts to the austenitic phase. Since this tends to occur by the individual martensite crystallites shearing back to the austenite crystals from which they were formed, the original specimen shape is recovered. However, this is only possible if all of the deformation (apart from that due to conventional elasticity) has been achieved by martensitic phase transformations. If an excessive strain is imposed, then it is likely that some conventional plastic flow (dislocation glide) will occur, and of course this will be irreversible.
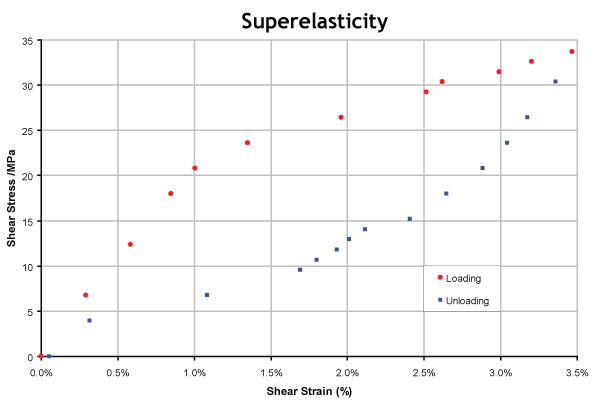
Superelasticity - Hysteresis in the Stress-Strain Behaviour
Stress-strain plots of SE alloys exhibit pronounced hysteresis, since the reverse (martensite- austenite) transformation does not occur at the same stress levels during unloading as the forward transformation did during loading. This is analogous to the hysteresis observed during thermal cycling, and occurs for the same reasons - ie extra driving force is required due to the stored elastic strain energy contribution.
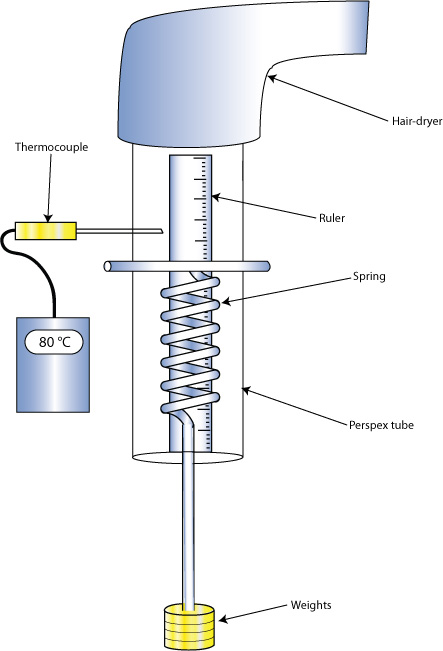
Diagram of apparatus to investigate superelasticity of a Ni-Ti spring
To investigate superelasticity a coil of Ni- 43at%Ti wire was held at a temperature above it’s Af temperature inside a perspex tube and was then loaded and unloaded with the associated extensions recorded. Superelastic behaviour can readily be illustrated using a specimen in the form of a spring. This geometry is very convenient for such purposes, since it allows large macroscopic extensions to be generated, while the local strain (which is pure shear) remains relatively low. The relationship between local and macroscopic strains, and the role of wire diameter and spring diameter, are fully explained here..
This clearly shows the hysteresis expected.
From the initial (linear) relationship between stress and strain , the Young’s Modulus of the austenitic phase can be obtained. The gradient gives the shear modulus, which can be converted to the Young’s Modulus using
Where G is the shear modulus, E is the Young modulus and ν is the Poisson ratio, for Nitinol ν ~ 0.3.
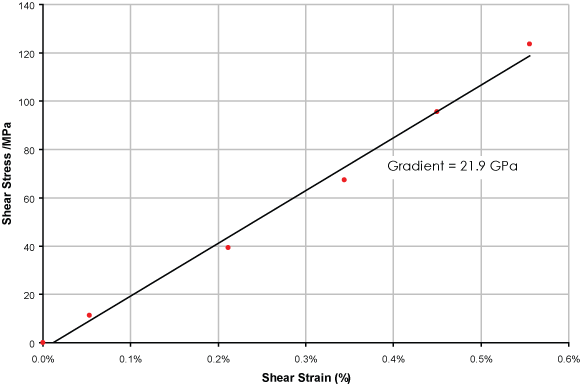
This leads to a value for E of about 60 GPa, which is in the range expected.
Shape Memory Effect - "Training" of the Transformation
The shape memory effect also involves martensitic transformations, but in this case they are stimulated, not by imposed mechanical strain, but by changes in temperature. It also involves the material being “trained” to have a preferred shape. This is done in the following way.
The component is given a thermo-mechanical treatment, which involves holding at a high temperature (usually well above Af), followed by cooling (to below Mf), while mechanically constrained to have a particular shape - eg a spring. Stress relaxation occurs during the holding period and then, during cooling (in the constrained shape), the austenite-martensite transformation takes place in such a way as to minimise the overall shape change. When a portion of the austenitic lattice shears so as to form the martensitic phase, there are usually several alternative directions in which it can do this – forming what are often termed different “variants” – so it’s possible for groups of variants to be formed which, taken together, have a very similar shape to the original parent material. This training predisposes the component to adopt the shape concerned when the phase transformation occurs, since this minimises the associated elastic strain energy.
Once a component has been “trained” in this way, then, after it has been deformed in an arbitrary way, it can recover its “trained” shape just by reheating it to above its Af temperature
Video of spring being distorted at room temperature, on heating it returns to its original shape
It’s also possible, using slightly more complex thermo-mechanical treatments, to create components which exhibit a “Two-way shape memory effect”, such that they can be cycled between two pre-determined shapes by thermal cycling. Such components are used in devices such as those designed to automatically open greenhouse windows in hot weather, and close them when it gets cooler.
Shape Memory Effect - The "Ferris Wheel" Experiment
An illustration of a shape memory effect is provided by the “ferris wheel” set-up shown in the accompanying video and simulation. Ten “trained” nitinol springs comprise the periphery of the wheel, connecting ten thin steel spokes, via relatively massive brass weights attached at their ends. The “trained“, shape of these springs, which is promoted by heating, is the contracted form. As individual springs cool, on the other hand, the forces acting between them tend to stretch them out again.
If the springs on one side of the wheel are heated, for example with a simple fan or radiant heater, then they tend to contract - ie to adopt the trained shape, as explained on the previous page. This bends the adjacent spokes, so as to move the brass weights towards the side where the heater is located. This creates a net moment tending to rotate the wheel. Exactly how the wheel rotates depends on the heating and cooling characteristics, and also on the transformation characteristics of the SME and on the dimensions (wire and spring diameters) of the springs.
Watch a video of the actual wheel and then see what happens to the individual springs when they are heated and cooled
Video of wheel rotating on heating
Heating
Close up video of wheel springs heating
Cooling
Close up video of wheel springs cooling
Microstructural Changes during Thermo-Mechanical Treatment
The martensitic phase transformations taking place during Superelastic and Shape Memory behaviour cause characteristic changes in the microstructure. These are particularly striking if viewed dynamically, when the nature of the shear displacements taking place can often be seen very clearly. This is assisted by using viewing conditions such that the martensitic and austenitic phases are readily distinguishable.
The two videos available here show:
1) a martensitic specimen being mechanically compressed, inducing two sequential changes of the orientation of the crystal by twinning.
2) a specimen being cooled, and then heated, inducing transformation to martensite and then reversion to the austenitic phase.
In both cases, the specimens are being viewed by optical microscopy, using Nomarski differential interference contrast. The width of the viewed area is in both cases about 200 µm.
Video 1:
Video of a martensitic specimen being mechanically compressed, inducing two sequential
changes of the orientation of the crystal by twinning
A CuAlNi single crystal (2H orthorhombic phase ) is compressed (vertical axis) at room temperature, causing activation of two sequential twinning deformations. As austenite, the crystal is cube-shaped, whereas in the martensite form it is sheared. Six different sheared martensite crystals, having well - defined prism shapes (three of which appear in this video), can be created by pressing on 3 different faces of the cube . . It is essential that the loading arrangement allows lateral displacements to occur.
Video 2:
Video of a specimen being cooled, and then heated, inducing transformation to martensite
and then reversion to the austenitic phase
A bi-crystal of austenitic CuAlNi is cooled, causing transformation to the martensitic (2H orthorhombic) phase. The process is reversed in the second half of the video, as the specimen is heated again. The rate at which transformation occurs is controlled by heat flow effects. (The shear process itself tends to take place very rapidly.) The martensitic phase is internally twinned. This is very clear within the dark-coloured phase moving in from the left-hand side in the first part of this video.
These videos are made available by the courtesy of Prof. Vaclav Novak and Prof. Petr Sittner, from the Department of Functional Materials, in the Institute of Physics of the ASCR, Prague, Czech Republic. Further technical details are available in the following publication:
V. Novak, P. Sittner, S. Ignacova, T. Cernoch, Transformation behavior of prism-shaped shape memory alloy single crystals , Mat Sci and Eng A , 438-440 (2006) p.755-762.
Limits of Superelasticity
There are limits to the temperature and stress ranges within which superelastic deformation can occur. The stress needed to initiate the austenite-martensite transformation rises with increasing temperature (ie as the austenite phase becomes thermodynamically more stable). This dependence is predicted by a form of the Clausius-Clapeyron equation. \[\frac{{{\rm{d}}\sigma }}{{{\rm{d}}{M_{\rm{s}}}}} = \frac{{ - \Delta H}}{{T{\varepsilon _0}}}\]
where ΔH is the latent heat of the transformation and ε0 is the associated strain. {this eqn. needs checking } At temperatures well above the stress-free value of Ms, quite substantial stresses may be needed to stimulate martensite formation. Furthermore, the stress needed to induce dislocation motion (in the austenite phase) is likely to fall with increasing temperature.
The temperature at which these two processes (slip and martensite formation) require the same applied stress will be the upper limit for both superelastic deformation and the shape memory effect (since slip will occur preferentially above this temperature). Superelastic behaviour requires a minimum temperature of Af, since the specimen should be fully austenitic initially. The shape memory effect not only requires heating to above Af, but also cooling down to Mf
Therefore SE occurs at temperatures between Ms and Md where Md is the temperature at which slip becomes easier than the formation of the martensitic phase.
Others limits
It’s also possible for local defects to accumulate during repeated transformation, which can reduce the achievable strain and the force that can be exerted by the transformations. Also, excessive deformation, beyond that which can be accommodated by transformation to martensite, will lead to irreversible strain (plastic deformation by slip).
Applications
The most widely used shape memory alloy is the equi-atomic Nickel Titanium alloy known commercially as Nitinol.
Superelasticity
Superelastic stents are used to hold open arteries or other vessels. They can be tightly compressed while being guided into the body then, when released, they spring back to their larger shape.
They are also used to hold together broken bones. Conventional pins need to be tightened as bone heals, which either involves further operations or an external framework. Superelastic devices, on the other hand, contract as the bone heals and provide guiding pressure, forcing the bones back to the correct shape.
Other uses include spectacle frames and brassiere wires. If superelastic material is bent out of shape, it quickly returns to its original shape.
Shape memory effect
Shape memory effects are used in actuators , to produce motion in response to temperature changes. A simple example is a greenhouse window, which automatically opens and closes in response to temperature changes.
Other examples include the new Boeing 787 where small chevrons on the trailing edge of the engine move with varying temperature.
On take off the engine is hotter and they move into a position which makes the engine run more quietly. However once away from the airport the engine cools in higher air and the flaps move to give better fuel economy.
Another example is in clips used to hold solar panels in place on both the Hubble Space Telescope and the International Space Station. When cold they hold the panels closed up, but when out in space when the panels heat due to the sun the clips undo and allow the panels to unravel to their full size.
These mechanical solutions are preferred to electrical systems because they are more reliable as there are fewer things to go wrong.
Summary
An outline has been given of how displacive (shear) transformations can effect a macroscopic shape change. These transformations occur by the cooperative, systematic motion of all the atoms in the region concerned by small distances with respect to their neighbours. Unlike a similar shape change generated by conventional plasticity (dislocation glide), such transformations, and hence the shape change, can be reversed. This can occur by simply removing the applied stress, giving rise to so-called Superelasticity. Shape changes can also be stimulated by changing the temperature, thus altering the relative stability of the two phases. This behaviour is exploited in the Shape Memory Effect, in which defined shape changes are induced by changing the temperature, either just recovering a prescribed shape after mechanical deformation or cycling between two defined shapes by thermal cycling.
Questions
Quick questions
You should be able to answer these questions without too much difficulty after studying this TLP. If not, then you should go through it again!
-
Why is a martensitic transformation often termed "displacive"?
-
Which of the following statements are correct concerning displacive (martensitic) and diffusional phase transformations?
-
Martensitic transformations often exhibit hysteresis - for example, the temperature must be taken considerably above that at which the two phases have the same free energies during heating, in order for the transformation to go to completion, whereas it needs to be cooled well below that temperature in order for it to fully reverse. Which of the following explanations for this effect is correct?
-
Unlike loading and unloading of a specimen to and from its conventional elastic limit, doing this to a superelastic material, to and from its superelastic limit, leads to energy being (permanently) absorbed within the specimen, despite the fact that the original specimen shape has been recovered. Assuming "ideal" superelastic behaviour, which of the following could happen to this energy?
Deeper questions
The following questions require some thought and reaching the answer may require you to think beyond the contents of this TLP.
-
A component, to be made from a NiTi Shape Memory Alloy, must be superelastic under service conditions. Thermal cycling, while monitoring the phases present, gave the plot below. Thermodynamic calculations indicate that the stress level to stimulate martensite formation rises with temperature at 1 MPa K−1. The flow stress (for dislocation glide) is 100 MPa at 20 °C and falls with increasing temperature at 0.3 MPa K−1. Calculate the maximum use T.
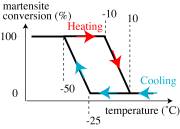
Dependence of phase proportion on temperature during (unloaded) thermal cycling
Going further
Books
There are relatively few books specifically on this topic, and most of these are compilations of chapters at the research level. It is, therefore, currently rather difficult to identify a coherent, introductory level book dedicated to the topic, however, a good starting point may be "Smart Structures: Analysis and Design" by A. V. Srinivasan, D. Michael McFarland, CUP, 2000.
Websites
There are a number of websites giving various types of information. A good starting point would be that of the Department of Functional Materials at the Czech Academy of Sciences, where Prof. Petr Sittner is based - see http://department.fzu.cz/ofm/wwwOFM/index.php?lang=en. Various videos and illustrations of shape memory devices etc are available there.
Deformation Twinning
Since in twinning only a small movement of atoms occurs and it is a co-operative process it can occur much more quickly than slip, making it much more common in high stress rate situations; for example, they are often seen when a material has exploded. Also they are preferentially formed when there are few slip systems available, which is the case in low symmetry crystals. This is why they occur readily in shape memory alloys which are usually hcp or monoclinic.
Clausius-Clapeyron
The Clausius-Clapeyron equation in its most common form is used to determine the gradient of phase transition lines on a pressure-temperature plot.
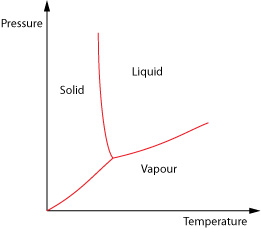
To derive the Clausius-Clapeyron equation we look at how pressure and volume vary with each other as a phase change occurs. We shall consider the liquid-gas change.
We need to form a heat engine cycle known as a Carnot Cycle. To do this we plot how the pressure and volume of the material vary when the temperature is held constant at two different temperatures (isothermals) and then add two adiabatic curves which show the relationship when no heat is allowed to flow in or out of the materials.
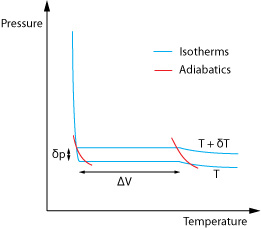
This forms the closed cycle which as we move round takes in and releases energy and is defined to have efficiency η, where;
\[\eta = \frac{W}{{\Delta H}} = \frac{{\delta T}}{T}\]
ΔH is the latent heat and is given as ΔH = T ΔS where ΔS is the associated change in entropy. The work done in the cycle, W, is given by its area, W = δp ΔV so;
\[\frac{{\delta T}}{T} = \frac{{\delta p\Delta V}}{{T\Delta S}}\]
and so as δT → 0 the gradient of the line showing the coexistence of the two phases on a plot of p vs T is given by;
\[\frac{{\delta P}}{{\delta T}} = \frac{{\Delta S}}{{\Delta V}} = \frac{{\Delta H}}{{T\Delta V}}\]
ΔH is the latent heat of the process, T is the temperature and ΔV is the associated volume change with the process.
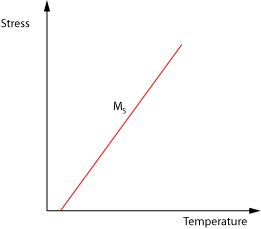
We however wish to know how the stress required to induce the martensitic change varies with temperature so we use the form;
\[\frac{{{\rm{d}}\sigma }}{{{\rm{d}}{M_s}}} = \frac{{ - \Delta H}}{{T{\varepsilon _0}}}\]
Where σ is the applied stress, Ms is the shifted temperature, and ε0 is the transformation strain along the direction of applied stress.
Displacive v diffusive phase transformations
Martensitic transformations are very different from those involving diffusion of atoms, i.e. reconstructive transformations. In martensitic transformations, the atoms move in an organised manner relative to their neighbours. This homogeneous shearing of the parent phase creates a new crystal structure, without any compositional change (no diffusion). Martensitic transformations are also known as “diffusionless”, “displacive” or “military”. The difference between displacive and diffusional transformations is demonstrated in the following animation;
Academic consultant: Bill Clyne (University of Cambridge)
Content development: Dave Bosworth (2007) and Andy London (2009)
Photography and video: Brian Barber
Web development: Lianne Sallows and David Brook
This DoITPoMS TLP was funded by the UK Centre for Materials Education and the Department of Materials Science and Metallurgy, University of Cambridge.
Additional support for the development of this TLP came from the Worshipful Company of Armourers and Brasiers'.