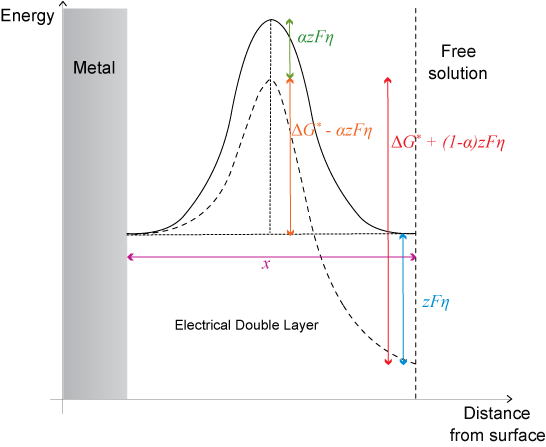
The Energy Landscape
Under equilibrium conditions, the energy landscape is symmetrical when free energy is plotted against distance from metallic surface:
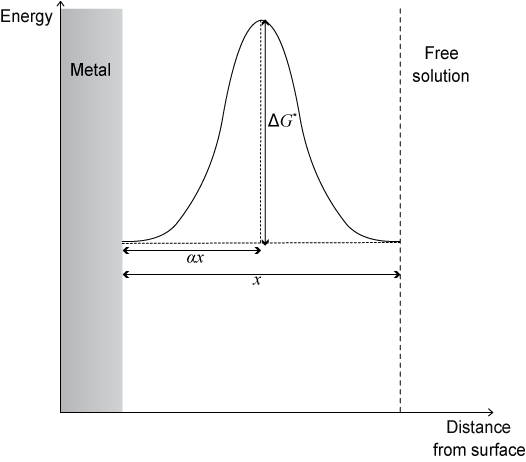
The fraction of the width of the double layer that must be crossed to reach the excited state is known as the symmetry factor, α.
However, when overpotential is applied, the energy is changed on the free solution side of the plot by an amount -zFη. The overpotential is distributed so that a fraction, α lies across the barrier in the forward direction and (1 − α) lies across the barrier in the backward direction.
The overall effect of the overpotential is to lower activation energy for the forward reaction by α z Fη. Thus the Arrhenius relation now becomes:
\[{k^′} = {k_0}\;\exp \left( {\frac{{ - (\Delta {G^0} - \alpha zF\eta )}}{{RT}}} \right)\]
\[{k^′} = k\;\exp \left( {\frac{{\alpha zF\eta }}{{RT}}} \right)\]
where k’ is the new rate, k is the rate without the overpotential.