Derivation of Fick's first law
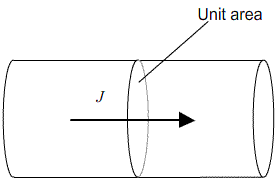
Fick’s first law relates this concentration gradient to the flux, J, of atoms within the crystal (that is, the number of atoms passing through unit area in unit time)
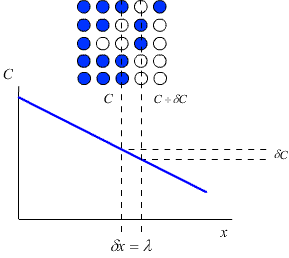
Each plane within a crystal will contain Cλ atoms per unit area.
This means that for an increment of distance (δx) of λ, the corresponding increment of concentration (δC) is given by:
\[\partial C = \lambda \left\{ {\frac{{\partial C}}{{\partial x}}} \right\}\]
For a three-dimensional crystal, an atom can move in one of six directions. If the jump frequency is ν, the fluxes of atoms from left to right and from right to left are given by:
\[{J_{L \to R}} = \frac{1}{6}\nu C\lambda \] \[{J_{R \to L}} = \frac{1}{6}\nu (C + \partial C)\lambda \]
Therfore the net flux, J, is given by:
\[ J = {J_{L \to R}} - {J_{R \to L}}\] \[J = - \frac{1}{6}\nu \partial C\lambda \] \[J = - \frac{1}{6}\nu \left\{ {\frac{{\partial C}}{{\partial x}}} \right\}{\lambda ^2}\] \[J \equiv - D\left\{ {\frac{{\partial C}}{{\partial x}}} \right\}\]
This is Fick’s first law: D is the diffusivity of the diffusing species.

