Applications
We shall consider the following reactions on the Ellingham diagram below: the
oxidation of silver to form Ag2O (s); and the oxidation
of cobalt to form CoO (s) and Co3O4:
4Ag (s) + O2 = 2Ag2O (s);
2Co + O2 (g) = 2CoO (s)
3Co + 2O2 (g) = Co3O4 (s).
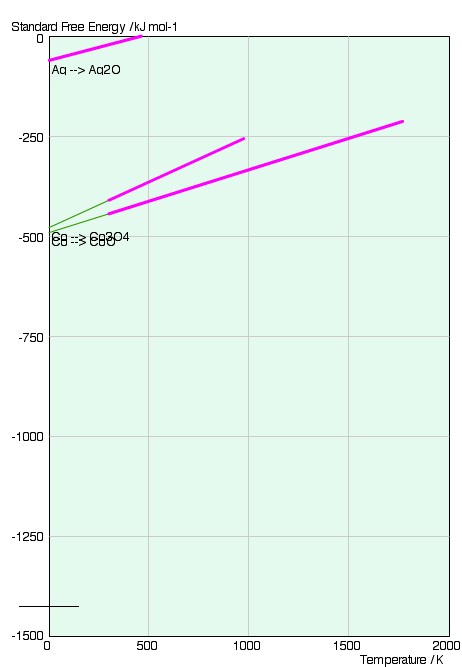
The graph consists of lines of
$$\Delta G^\circ = \Delta H^\circ - T\Delta S^\circ $$
Although ΔS and ΔH vary with temperature (see here to see why), the changes are so small as to be negligible, and so the lines are approximately straight. This means that they are of the form
$$y = mx + c$$
We can immediately see that the standard free energy change is greater (more negative) for the cobalt reaction relative to that of silver at all temperatures. This means that at all temperatures the equilibrium constant is larger for the cobalt reaction-the composition is further weighted towards the products of the reaction.
This is the reason that metals that appear higher up on the diagram are more stable than those metals that appear lower down, and are more likely to be found in their pure solid form. The gradient of the two lines is approximately the same.
We can see that the gradient of the lines is simply the standard entropy change for the reactions;
$${{\partial \Delta G^\circ } \over {\partial T}} = - \Delta S^\circ $$
This is evident from the reactions, which both involve the elimination of one mole of gas - a large decrease in entropy. This is the reason for the positive slope of the lines.
The reason for the change in slope is the change in phase of a component of the system, which alters the entropy change.
As the standard free energy change for both reactions is still negative (below 460K) the large decrease in entropy must be counteracted by a large enthalpy of reaction. This is indeed the case. The intercept of the lines with 0K gives the enthalpy of the reaction:
$${\left. {\Delta G^\circ } \right|_{0K}} = \Delta H^\circ $$
We can hence see that the relative stability of the oxide of cobalt compared with the oxide of silver is due to the much larger standard enthalpy of reaction.

