Why are only some materials ferromagnetic?
For ferromagnetism to occur there must be an internal driving force that causes parallel alignment of the spins of the electrons to be the more favourable state. Weiss explained this observed behaviour by proposing the presence of an internal interaction between the localised moments called a molecular field. Whilst Weiss’ phenomenological model represented a significant step forward, the microscopic explanation of his molecular field required the laws of quantum mechanics. The important term in the interaction of the localised moments is called the exchange interaction.
The exchange interaction occurs due to the Pauli Exclusion Principle; if two electrons have different spins then they can occupy the same orbital (angular momentum state), hence be closer to each other and they will therefore have a stronger Coulomb repulsion.
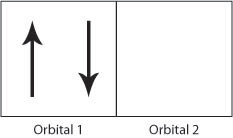
Figure H. Schematic showing two electrons occupying orbital 1 producing a strong Coulomb repulsion compared to Figure I.
If the electrons have the same spin then they will occupy different orbitals and therefore have less Coulomb repulsion as they will be further apart. In this way, the exchange energy (the energy due to the repulsion between the two electrons) is minimsed.
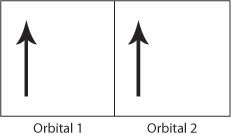
Therefore the Coulomb repulsion force favours the parallel alignment of all the electron spins as the exchange energy is minimised. The above rules help to explain the strong ferromagnetic order seen in iron, cobalt and nickel. The Flash demo below allows the electronic structure of the transition elements in the 3rd row of the periodic table and the numbers of unpaired electrons to be seen.
Taking the elements shown in the demo above as an example, it is, however, not straightforward to predict the presence or form of magnetic order from the electronic configuration alone as not all elements with unpaired electrons are ferromagnetic. There are other factors, principally the atomic structure, that go towards determining the type of magnetism exhibited by the elements. See the discussion on band theory in reference 1.
This theory can be summarised into rules:
- In the elements, electrons maximise their total spin and so will occupy orbitals with one electron per orbital with all the spins parallel until all the available orbitals contain an electron. Once this has occurred the electrons pair up in these orbitals in pairs of opposite spin.
- For atoms with filled electron shells the total atomic orbital angular momentum and total spin are zero; therefore there is no magnetic dipole moment.
- For atoms with incomplete outer shells, only the incomplete shells need to be considered in calculating the total angular momentum and hence magnetic dipole moment.
- If an atom has no incomplete shells then there is no permanent dipole. These atoms are called diamagnetic.
- Materials that do contain unpaired electrons are ferromagnetic only if the atomic structure allows the cooperative parallel alignment of the magnetic dipole moments.

