Other types of fuel cells?
A fuel cell is defined as an electrochemical device that converts chemical energy to electrical energy (and heat) as long as reactants are supplied to its electrodes. In contrast to batteries, this implies that neither the electrodes or the electrolyte are used up by the operation of the cell, hence the fuel cell should keep running (wear and tear excepted) as long as fuel is supplied. These examples of “fuel cells” don’t all fulfil this criterion but are commonly mislabelled anyway.
- A “biological fuel cell” is still at the very early stages of development but shows promise in the long term. They would use an organic fuel such as natural gas or an alcohol. The key feature of this type of cell is that they would use enzymes rather than chemical catalysts (e.g. Pt) to facilitate the electrode reactions. This idea seeks to replicate nature, but it’s a long way from practical application.
- Metal/air cells such as the zinc air battery use an alkaline salt solution to act as an electrolyte with OH– as the charge carrier. At the anode, zinc is slowly corroded away to zinc oxide in the solution. The cell could be “refuelled” by adding more zinc to the electrode, but the fact that electrodes are consumed means that this is a battery rather than a fuel cell. Such cells do have high energy density and are commonly used for long life, low power applications. They are often called fuel cells because the cathodic reaction is identical to that in the fuel cell.
 Wind turbine
Wind turbine
Britain is the windiest country in Europe, so why are we not using wind turbines to supply a larger portion of our energy needs? The problem is that wind is a gusty phenomenon that is intrinsically inconsistent. Energy storage on a large scale must be a realisable technology before such energy sources can be utilised. Redox fuel cells are being developed as a means of storing energy.

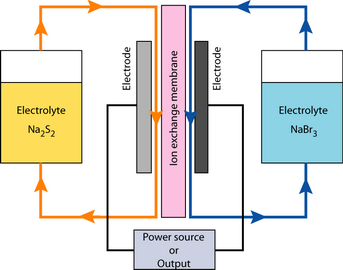
A Regenesys plant which was under construction near Cambridge (Left) and the principle behind a Redox fuel cell (Right).
Two different solutions are contained in two tanks. When energy is required, a solution of Na2S2 is fed to the anode, and NaBr3 to the cathode. The anodic reaction is:
2Na2S2 → Na2S4 + 2Na+ + 2e– |
The electrons flow around the external circuit so that at the positive electrode, or more precisely the cathode (remember, the definition is that the anode is the site of oxidation), we see:
NaBr3 + 2Na+ + 2e–→ 3NaBr |
As energy is drawn from the system, the sodium sulphide becomes sodium polysulphide and the tribromide becomes sodium bromide. This reaction is reversed when current is supplied to the cells. The system is called a fuel cell because the electrodes are just a surface for the reaction, and are not consumed by it. They are also fed and energy containing liquid. The electrolytes however are not really fuels and they are changed during the process. This system should probably be called a strange-battery.
- Proton Exchange Ceramic (PEM) fuel cells do exactly what they say on the tin. They will utilise fossil fuels directly, at around 700 °C, reducing them at the anode and conducting protons across a ceramic electrolyte. They are at the very early stages of development, but potentially have a great advantage over the low temperature PEM cells in that high temperature exhaust gases can be used in a turbine hybrid system to generate more electricity and push the system’s efficiency up to around 80%.

