Materials for constructing bipolar plates
Materials used and manufacturing techniques vary a lot between producers. One thing many manufacturers have in common is that the plates are often produced in two halves and stuck together afterwards. This makes it easier to have cooling fluid channels running through the body of the plates as instead of drilling holes through them, one can mill away the surface of two half plates and then combine them.
Some manufacturing techniques and materials (the two are usually intrinsically linked) are outlined below:
![]()
Graphite based
- Graphite sheet machined with channels
Advantages:
- Easily machined by automated processes
- Light weight material
- Good conductive material
Disadvantages:
- Graphite is porous and therefore the plates need to be relatively thick.
- Whilst the machining of channels is a fairly easy process, it is one that takes a long time – considerably increasing the production cycle time.
- Graphite is brittle meaning that the plates must be handled carefully – making assembly difficult.
- Injection moulded graphite filled polymer resins
Advantages:
- Very cheap technique
- Short production cycle
Disadvantages:
- The polymers aren’t generally conductive enough to be useful, however developers working on these techniques recently, with epoxy resins and course graphite, claim 75% graphite content and through plane conductivity of 20 S cm-1. This technique could become useful in the future.
- Compression moulded graphite particles in a thermoplastic polymer:
This technique involves a mixture of graphite and thermoplastic granules in a mould, heated above the glass transition temperature (Tg) of the polymer under pressure until the materials mix together and flow into the mould.
Advantages:
- Can use a higher proportion of carbon particles than in the injection moulded method meaning conductivity is greater.
- The technique and the machinery involved are relatively simple.
Disadvantages:
- Very slow production cycle (~15 min) as the mould must be cooled before each half plate can be removed.
- Carbon-carbon composites:
There are a couple of ways of using carbon-carbon composites in bipolar plates. The complex channels of the plate are formed by moulding a carbon composite in one of the two ways outlined below, but this alone doesn’t produce a plate that is strong enough, flat enough or impermeable enough to meet our specifications (basically too flimsy). A layer of solid carbon is applied to the back of this plate to improve properties.
One possible process involves the graphitisation of a carbon powder in a mould by heating it to 2500 ºC. This is expensive and requires a very precise control of temperature. It does have the advantage of relative simplicity of concept. The alternate method used is to mould the channels from a suspension of short carbon fibres in a phenol resin, which is then cured at 150 ºC for a few minutes to give a very high conductivity (>200 S cm-1) but high porosity plate. The back of this can then be made hermetic by depositing a layer of solid carbon via chemical vapour deposition.
Advantages:
- High stiffness
- High conductivity
- Low permeability
- Can be done quickly
Disadvantages:
- Quite expensive materials involved
![]()
Metal based
Metals have several advantages over carbon based plates:
- Much higher conductivity of heat and electricity
- Very easy machining (e.g. stamping)
- Non porous (can be made thin)
- High modulus
- Can be low cost
However, the major disadvantage is their susceptibility to corrosion. The fuel cell environment is hostile. To protect a metal plate from corrosion requires some sort of treatment that would negate one or more of the advantages of using metal plates in the first place; for example, stainless steel plates could be used, which would be resistant to corrosion but which are expensive to produce and difficult to machine. Coatings are a practical possibility, but add to the cost again.
The use of metals in bipolar plates does however make way for some novel designs. The image below is of titanium foamed with argon. Titanium is a poor conductor compared to other metals, but it is still 30 times more conductive than graphite. Metallic foams allow great electrical contact with the whole surface of the cell, and at the same time allow gases to get to the whole surface. The foam does however have a tendency to clog with product water. One approach to this problem involves impregnating areas of the foam with epoxy or even simply crushing areas of it, so as to create channels of porosity in a similar way to the channels cut in carbon plates.
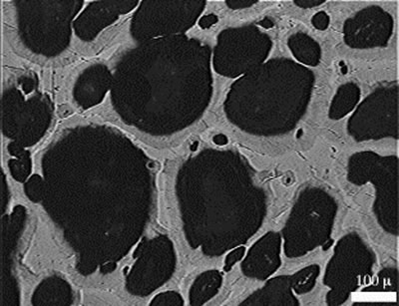
Titanium foamed with argon (Source: North Western Edu)
A major selling point of this metal foam approach to the bipolar plate is the simplicity of joining the components. The foam can be produced in blocks and then sliced like cheese. Add a thin sheet of impermeable metal between two slices of foam, surround it all with a gas-tight seal and add the connecting tubes and you have an effective bipolar plate. Titanium is also an easily protected material by means of nitriding the surface.

