Re-use of this resource is governed by a Creative Commons
Attribution-
NonCommercial-ShareAlike 4.0 International
https://creativecommons.org/licenses/by-nc-sa/4.0/
NonCommercial-ShareAlike 4.0 International
https://creativecommons.org/licenses/by-nc-sa/4.0/
An interactive phase diagram, for the Fe-C system.
To use, roll over the region of the phase diagram which
interests you.
interests you.
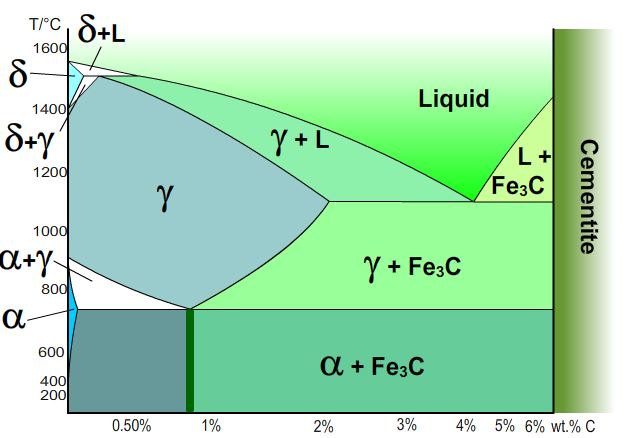
Rollover different sections of the phase diagram
for more information.
Austenite:
Above certain temperatures iron and carbon are in solid solution. This is austenite which has a cubic close-packed (or face-centred cubic) structure. Alloying additions (such as Mn, Mo, Cr and Ni) stabilise austenite at lower temperatures, so austenitic steel can be found at room temperature.
Austenite is differentiated from ferrite by the variation in shade between grains, and the presence of annealing twins. It is the only common non-magnetic phase of steel.
Above certain temperatures iron and carbon are in solid solution. This is austenite which has a cubic close-packed (or face-centred cubic) structure. Alloying additions (such as Mn, Mo, Cr and Ni) stabilise austenite at lower temperatures, so austenitic steel can be found at room temperature.
Austenite is differentiated from ferrite by the variation in shade between grains, and the presence of annealing twins. It is the only common non-magnetic phase of steel.
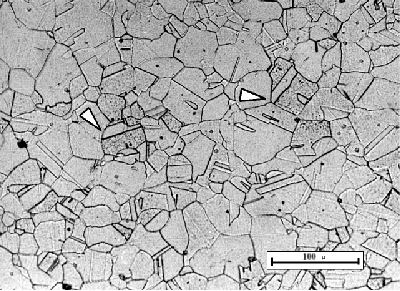
Click
the micrograph to see a schematic sketch.
Rollover features for a description
Eutectoid composition:
A steel of composition Fe-0.8 wt.%C will undergo a eutectoid reaction in which austenite transforms to a mixture of ferrite and cementite below a certain temperature (~700°C). A eutectoid reaction involves the transformation of one solid phase into another two solid phases.
The resulting fine lamellar intergrowth of ferrite and cementite is called pearlite.
A steel of composition Fe-0.8 wt.%C will undergo a eutectoid reaction in which austenite transforms to a mixture of ferrite and cementite below a certain temperature (~700°C). A eutectoid reaction involves the transformation of one solid phase into another two solid phases.
The resulting fine lamellar intergrowth of ferrite and cementite is called pearlite.
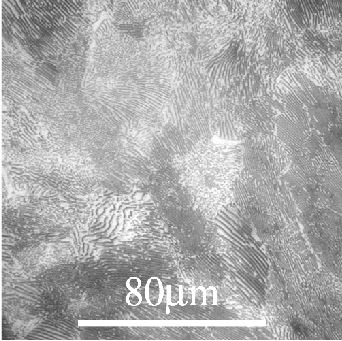
Pearlite:
Diffusion limits how far C can diffuse from the forming ferrite phase to Fe3C, so fine lamellae are seen.
Diffusion limits how far C can diffuse from the forming ferrite phase to Fe3C, so fine lamellae are seen.
Hover over the different phases in this sketch.
C rejected from ferrite phase
C taken up by cementite phase
Hypereutectoid
The cementite phase precipitates first here, carbon diffusing out of the austenite changing the composition. This continues until the eutectic composition is reached and the remaining austenite transforms to pearlite.
The cementite phase precipitates first here, carbon diffusing out of the austenite changing the composition. This continues until the eutectic composition is reached and the remaining austenite transforms to pearlite.
In this micrograph the cementite (Fe3C) is
pale and can be seen between pearlite grains.
Ferrite:
Ferrite has a body centred cubic structure with a small amount of C in solid solution. It is often allotriomorphic, meaning it nucleates preferentially on the austenite grain boundaries, so the grains do not reflect the crystalline symmetry. Growth can be directed along crystal planes in the austenite giving a Widmanstätten pattern.
Ferrite has a body centred cubic structure with a small amount of C in solid solution. It is often allotriomorphic, meaning it nucleates preferentially on the austenite grain boundaries, so the grains do not reflect the crystalline symmetry. Growth can be directed along crystal planes in the austenite giving a Widmanstätten pattern.
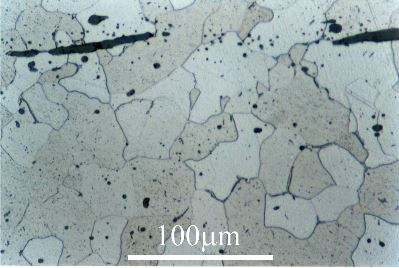
Ferrite has
no precipitates and relatively uniformly coloured grains.
In this micrograph there are impurities (black shapes).
In this micrograph there are impurities (black shapes).
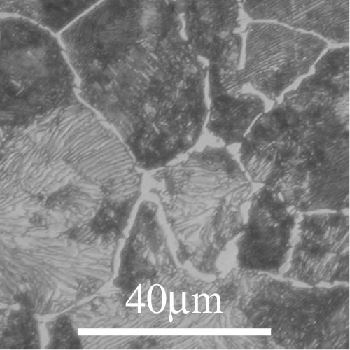
Hypoeutectoid:
In this section the ferrite begins to precipitate out first (on austenite grain boundaries). When the alloy reaches the eutectoid composition the remaining austenite transforms to pearlite (ferrite and cementite lamellae).
In this section the ferrite begins to precipitate out first (on austenite grain boundaries). When the alloy reaches the eutectoid composition the remaining austenite transforms to pearlite (ferrite and cementite lamellae).
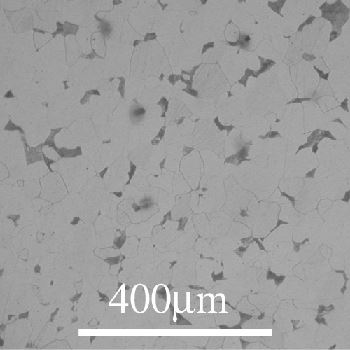
In this micrograph we see the pale ferrite grains with
areas of darker pearlite lamellae.
Roll over the micrograph to see a stronger magnification image.
Roll over the micrograph to see a stronger magnification image.
Cementite:
Cementite has chemical formula Fe3C and an orthorhombic lattice. It is very hard and brittle, due to the (relatively) high C content.
Cementite has chemical formula Fe3C and an orthorhombic lattice. It is very hard and brittle, due to the (relatively) high C content.
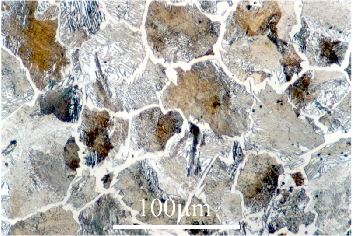
Cementite is highly reflective,
appearing very white under an optical microscope, allowing easy identification.
In this micrograph (hypereutectoid) the bright white strips between darker grains are cementite.
In this micrograph (hypereutectoid) the bright white strips between darker grains are cementite.