The Nernst Equation and Pourbaix Diagrams
AimsBefore you startIntroductionBackgroundThe Nernst equationConstruction of a Pourbaix DiagramAnatomy of a Pourbaix DiagramExamples of a Pourbaix DiagramConstructing a 3D Pourbaix DiagramSummaryQuestionsGoing furtherTLP creditsTLP contentsShow all contentViewing and downloading resourcesAbout the TLPsTerms of useFeedbackCredits Print this page
PreviousNext
Examples of a Pourbaix Diagram
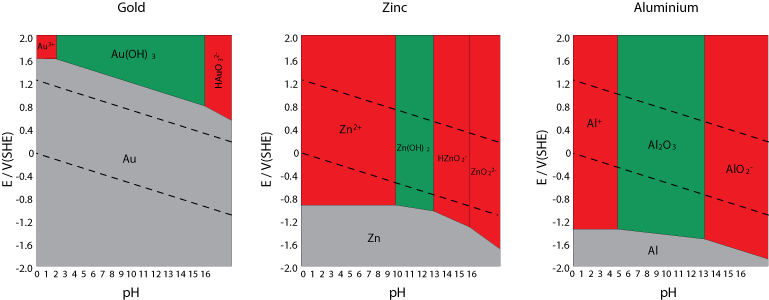
Gold’s Pourbaix diagram explains why it is the most immune substance known. It is immune in all regions in which cathodic reactions can take place. So gold never* corrodes in an aqueous environment.
Immunity of aluminium only occurs at lower potentials. Therefore, unless under conditions that cause it to passivate, it is much more susceptible to corrosion than gold or zinc.
* provided that the water is pure; that no ion complexes are present to provide a cathodic half cell reaction that occurs at a potential higher than +1.5 V(SHE).

