Pyroelectric Materials (all content)
Note: DoITPoMS Teaching and Learning Packages are intended to be used interactively at a computer! This print-friendly version of the TLP is provided for convenience, but does not display all the content of the TLP. For example, any video clips and answers to questions are missing. The formatting (page breaks, etc) of the printed version is unpredictable and highly dependent on your browser.
Contents
Aims
On completion of this TLP you should:
- Understand the difference between pyroelectrics and ferroelectrics.
- Understand the main uses of pyroelectrics.
- Understand the difficulties involved in using pyroelectrics, and how these issues are overcome.
Before you start
This TLP follows on well from those on ferroelectrics and piezoelectrics. It would be useful to read this after those.
Introduction
Pyroelectrics are the bridge between ferroelectrics and piezoelectrics – a Venn diagram showing the interrelations is available here.
They possess a spontaneous polarisation which is not necessarily switchable by an electric field. If their polarisation is electrically switchable then they are ferroelectric, a property that may be exploited e.g. for data storage. Pyroelectrics fill an entirely separate niche.
The pyroelectric effect has been known of for a very long time. The Greek philosopher Theophrastus first noted (in approximately 400 B.C.) that Tourmaline would attract straw and bits of wood when heated. This was due to the fact that the changing the temperature produces surface charges that are capable of attracting other charged materials. Naturally, how this occurred has only recently been discovered, but it is interesting to note how early on the effect was documented.
Polarisation
A pyroelectric material possesses a spontaneous dipole moment, interpreted via the ionic positions. This dipole moment, when normalised by volume, yields a polarisation. Whether a given sample with local dipole moments possesses a net dipole moment depends on domain configurations, which in turn depend on the electrical history of the sample. This polarisation can change when a stress is applied to the material, as pyroelectrics are a sub-set of piezoelectrics. But if the material is pyroelectric and not also ferroelectric then the polarisation will not reverse under the application of an electric field. This is because it will break down first, i.e. the coercive field exceeds the breakdown field. If the material is ferroelectric the the coercive field is smaller than the breakdown field. In other words, ferroelectrics are a subset of pyroelectrics.
Whether or not a material can be pyroelectric or ferroelectric depends upon whether the point group it belongs to is polar, i.e. whether there is at least one direction along which no point group symmetry element forces both sides of the crystal to be the same. The polar point groups are:
1, 2, m, mm2, 3, 3m, 4, 4mm, 6, 6mm. (Point groups will not be covered in this TLP.)
Note that in some of these point groups, e.g. class 4, the polar axis is unique.
Variation of Polarisation with Temperature
With a pyroelectric, the polarisation P will typically decrease when its temperature is raised. This is because the increasing disorder results in a reduced segregation of charge, and so the arising dipoles are lessened in magnitude. The drop off in polarisation can be seen on the next slide.
Here the polarisation drops off to the Curie point, where it is zero.
\[\underline {\Delta P} = \underline p \;\Delta T\]
where p = pyroelectric coefficient (C m-2 K-1). The pyroelectric coefficient is a vector, with three components:
\[\Delta {P_i} = {p_i}\Delta T\quad i = 1,2,3\]
Typically, however, the electrodes which measure this are placed along a principal crystallographic direction, and therefore, the coefficient is often measured as a scalar, which is typically negative to represent a polarization falling with increasing temperature.
The pyroelectric coefficient measured under an applied field E is liable to differ from its true value as explained below.
When an electric field, E is applied to a polar material,
a moment arises, and the total response D (as
measured as a charge per unit area on metallic plates either side of the pyroelectric)
is expressed as:
\[D = \varepsilon \;{\rm{E}} + {P_{\rm{s}}}\]
where ε = electrical permittivity of the pyroelectric and Ps = spontaneous polarisation.
This means that:
\[\frac{{\partial D}}{{\partial T}} = \frac{{\partial {P_s}}}{{\partial T}} + {\rm{E}}\frac{{\partial \varepsilon }}{{\partial T}}\]
Since the pyroelectric coefficient pg relates changes in D to changes in T, we have a ‘generalised’ pyroelectric coefficient, given by:
\[{p_{\rm{g}}} = \frac{{\partial D}}{{\partial T}} = \frac{{\partial {P_{\rm{s}}}}}{{\partial T}} + {\rm{E}}\frac{{\partial \varepsilon }}{{\partial T}} = p + {\rm{E}}\frac{{\partial \varepsilon }}{{\partial T}}\]
which includes a factor based on the permittivity of the material being temperature dependent. This is what is measured.
The true pyroelectric coefficient is given by:
\[p = \frac{{\partial {P_{\rm{s}}}}}{{\partial T}}\]
as this defines the variation of the spontaneous polarisation with T.
The effect due to changes in permittivity can sometimes be comparable in magnitude to true pyroelectricity, and can also be seen above the Curie point.
Behaviour around the Curie point
On warming towards the Curie point, above which the spontaneous polarisation of a pyroelectric disappears, the pyroelectric coefficient typically increases as the temperature dependence of the polarization becomes stronger.
The polarisation also depends on the order of the phase transition, covered in more detail here.
The diagram below demonstrates how the polarisation changes near the Curie point.
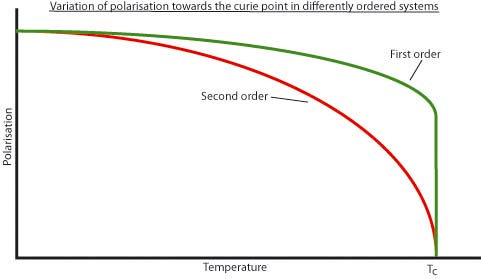
For second order transitions, the pyroelectric coefficient is observed to be large.
Materials which undergo first order transitions cannot be used for applications
as they undergo hysteresis, such that the transition occurs at different temperatures
depending on whether the material is being heated or cooled. This makes the
Curie point unreliable.
These factors mean that the pyroelectric is typically used at temperatures much lower than the Curie point. This results in pyroelectric coefficients being lower (as they are directly related to the temperature), but less variable with ambient temperature.
The Direct and Indirect Effect
As a pyroelectric material is also piezoelectric, thermal expansion can result in an indirect contribution to changes of polarisation, in addition to the direct effect discussed previously.
Example Pyroelectric Materials
Like other dielectric materials, the predominant pyroelectric structure is the perovskite.
Two examples are:
0.75Pb(Mg1/3-Nb2/3)O3-0.25PbTiO3, which has a pyroelectric coefficient of −1300 μC m−2 K−1 as a single crystal.
It is more commonly referred to as PMN-PT.
| |
Rotating crystal structure of PbMg/NbO3 |
and
Ba0.65Sr0.35TiO3, which has a pyroelectric coefficient of −7570 μC m−2 K−1.
It is also known as BST.
| |
Rotating crystal structure of Ba/SrTiO3 |
However, there are other types of pyroelectric material which do not have a perovskite structure. Two of the more common of these will also be considered.
Triglycine sulphate
This has the formula (NH2CH2COOH)3H2SO4, and variations on this have given some of the highest pyroelectric coefficients. The structure is shown below:
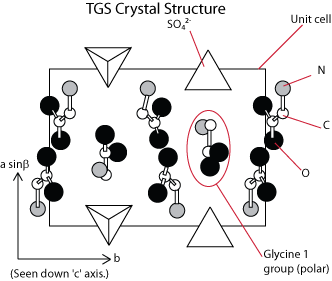
The glycine (NH2CH2COOH) groups are polar, but the most important is the glycine 1 group, as the reversal of the polarization of the material is associated with a rotation about the ‘a’ axis of this group. This changes the crystal into a mirror image of itself. In either state, below the Curie point, (~50oC) the crystal is point group 2, with a polar axis along the ‘b’ axis. Typically, the crystal is grown from aqueous solution, with whatever modifications are required. Modifications may include deuteration or more esoteric ideas such as substituting glycine for other amino acid style groups. These have altered properties such as Curie temp., or the thermal stability of the pyroelectric coefficient.
It has a pyroelectric coefficient of −5.5 × 10−4 C m−2 K−1, measured at 30oC. It is useful for the pyroelectric ‘vidicon’, a device used as a camera, for thermal imaging. This is used by disaster teams to find people trapped under rubble, etc.
Polyvinylidene fluoride
This is a carbon backbone polymer, with repeat unit (-CH2-CF2-). It takes up several different conformations, which possess different varying properties. The trans-gauche configuration results in molecules stacked so as to produce a non-polar unit cell, whereas the all-trans configuration results in a polar unit cell.
Possible configurations:
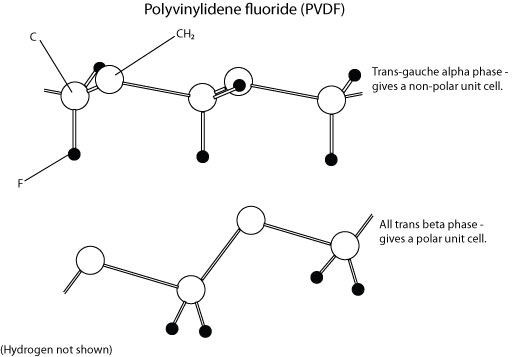
Unit cells:
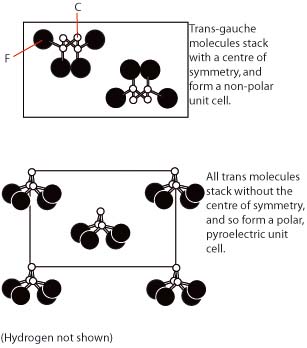
PVDF does not possess a particularly impressive pyroelectric coefficient, (−0.27 × 10−4 C m−2 K−1) but it is highly useful in that large area thin films can very easily be made. These structures are important, as they generate a large charge which can be easily detected. This overcomes most of the other failings of PVDF.
The large area thin film is most often used for measuring the energy of laser beams in laboratories, as a large area is required to detect the entire beam. Since it is also possible to very cheaply make small area thin films, PVDF is easily made use of in burglar alarms.
(This information sourced from; Pyroelectric devices and materials (review article), R W Whatmore, Rep. Prog. Phys. 49 (1986) 1335-1386, which provides plenty of good information on pyroelectrics.)
Application of a Pyroelectric-Infrared detection
There are problems with this situation. As discussed in the previous section, there is the possibility of an indirect effect, with the thermal expansion of the detector causing a polarisation to develop by piezoelectricity. This produces ‘noise’ of a sort, and can mask the signal generated by the target object. There is also the possibility of external stresses being applied. This is a problem, as it means the detector does not work as well as it should. These problems are typically counteracted by the use of a second pyroelectric component, i.e. a reference element.
See below:

Pollutant Control
Pollution is a very important issue. The reduction of pollution and greenhouse gases has become a major priority for parliament. We need to be able to monitor levels of pollution, in order to monitor the situation. This is where the pyroelectric comes in.
Pyroelectrics, as shown previously, are excellent detectors of IR radiation. Therefore, they make perfect devices for testing the level of IR radiation that passes through a gas sample. As the wavelength at which a gas absorbs usually uniquely identifies that gas, this makes an excellent means of detection.
See below for the mechanics of this:
Summary
As seen, the pyroelectric has somewhat limited use. However, it is a rather important type of material, due to the position it holds between piezoelectrics and ferroelectrics. It must also be considered that while it does not have a wide range of uses, its use in the motion detector (see the ‘Infrared Detection’ section) is very common, and as such it can be considered important to understand it.
Questions
Quick questions
You should be able to answer these questions without too much difficulty after studying this TLP. If not, then you should go through it again!
-
When the temperature of a pyroelectric is changed, where does the resultant charge imbalance arise?
-
Which of these is not a basic requirement for a pyroelectric?
-
What type of variable is polarisation?
-
Direct electrical measurement can necessarily identify what?
Going further
Books
A.J. Moulson & J.M. Herbert, Electroceramics: Materials, Properties,
Applications (2nd Ed., Wiley, 2003)
J.F. Nye, Physical Properties of Crystals (Oxford University Press)
R.E. Newnham, Structure-Property Relations (Springer-Verlag, 1975)
Academic consultant: Neil Mathur (University of Cambridge)
Content development: Robert Shaw
Photography and video: Brian Barber and Carol Best
Web development: Sam Wakeford, Robert Shaw and David Brook
This DoITPoMS TLP was funded by the UK Centre for Materials Education and the Department of Materials Science and Metallurgy, University of Cambridge.
Additional support for the development of this TLP came from the Worshipful Company of Armourers and Brasiers'