Stokes and anti-Stokes scattering
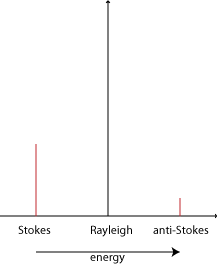
The difference in intensity of the Stokes and anti-Stokes components is due to the different number of molecules in each state initially. The population follows the Boltzmann distribution: \(N \propto \exp \)\(\left( { - {E \over {{k_B}T}}} \right)\)
Therefore there are exponentially fewer molecules that start out in the higher energy vibrational state. Since it is these that give rise to the anti-Stokes scattering, this is much less intense.
The disparity depends on the spacing of the energy levels. So for the less widely spaced rotational levels, the Stokes and anti-Stokes scattering are of similar magnitude. For the vibrational levels, which are spaced further apart, the anti-Stokes signal is significantly weaker than the Stokes signal.
The disparity is also reduced with increased temperature, and the difference can be used as a measure of temperature.

