Zone Refining
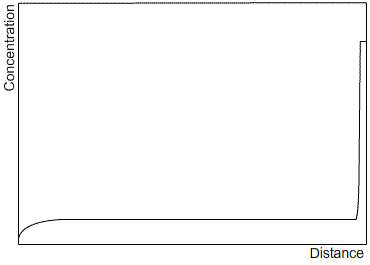
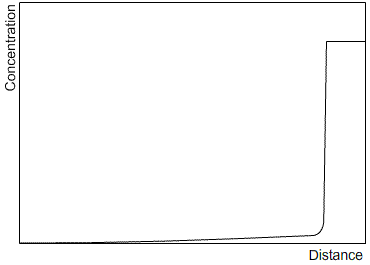
Zone refining is an industrial process which makes use of the fact that a material often solidifies with a higher purity than the surrounding liquid, in order to produce materials of very high purity. A bar is passed through a furnace that melts a small section of the bar. As the bar passes through the furnace, this section of liquid is passed along the length of the bar. The solid that forms as the section passes by is of a higher purity than the liquid, and the excess solute is partitioned into the liquid section. After one run, the solute profile will be similar to that after steady state solidification, as the limited size of the liquid section has the same effect as limiting the diffusion in the liquid. Subsequent runs will carry more of the solute to the end of the bar. After many cycles, almost all of the impurity will be concentrated at one end of the bar, which can then be removed, leaving a material of very high purity. Sketches of the solute profile of an initially homogeneous bar, which has been zone refined, are shown after one cycle (left) and many cycles (right).
 |
 |
This process is used in the production of silicon for use in the manufacture of microelectronic devices. The semi-conducting properties of silicon are highly dependent on the type and concentration of dopant atoms; so all impurities have to be removed before any dopants are added.

This picture shows the polished (left) and unpolished surfaces of silicon wafers cut from a bar that has been purified by zone refining.

