Dislocation interactions
Dislocation-dislocation interactions
Around a dislocation, the atoms are displaced from their normal positions. The atomic displacements are equivalent to that caused by elastic strains arising from external stresses. For example, the extra half plane of the edge dislocation puts the region above the slip plane into hydrostatic compression, whilst the region below the slip plane goes into tension.
When dislocations move under a tensile stress, their stress fields interact. As the elastic strain energy is proportional to the square of the local strain, it is energetically favourable for the stress fields to configure themselves to minimise this strain. The resultant configuration depends on the sign of the two dislocations interacting with each other. Dislocations of the same sign have the same burgers vector. Conversely, dislocations of opposite sign have opposite burgers vectors. (For more information about Burgers vector, see Introduction to Dislocations).
Below is an animation showing the attraction and repulsion of dislocations on the same and different slip plane
Dislocation-solute atoms interactions
Dislocations also interact with the solute atoms in a crystal. The solute atoms can be either interstitial or substitutional. The stress field created by the solute atom is spherically symmetric. The stress field is compressive if the solute atom is larger than the lattice atoms. On the other hand, the stress field is tensile if the solute atom is smaller than the lattice atoms.
The spherical symmetry of the stress fields induced by the substitutional solute atoms means the fields contain no shear stress component. Hence, they do not interact with screw dislocations, which are pure shear dislocations (i.e. screw dislocations have no hydrostatic tension or compression). However, the stress fields created by substitutional solute atoms will interact with the stress fields of edge dislocations. This will lead to favourable relative arrangements of dislocations and solute atoms, such that the strain energy is minimised.
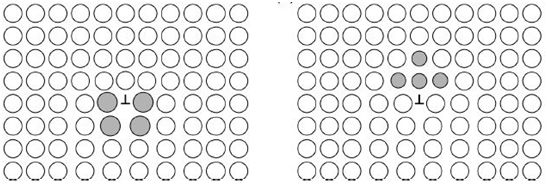
Figure 3: (Left) Larger solute atoms in the tensile field of an edge dislocation and (Right) smaller solute atoms in the compressive field of an edge dislocation. (Source of image: N. Jones, Course E: Mechanical Behaviours of Materials, Part 1A (2016), p. 60)
The interaction between dislocations and solute atoms leads to solute solution strengthening. If an edge dislocation interacts with a solute atom which is larger than the lattice atoms, the solute atom will reside below the extra half plane of atoms to relieve the hydrostatic tension (Figure 3). This causes the two stress fields to repel each other. On the other hand, if an edge dislocation interacts with a solute atom which is smaller than the lattice atoms, the solute atom will sit at the end of the extra half plane to relieve the hydrostatic compression. In both cases, an energetically favourable arrangement of the dislocation and the solute atom is formed, which tends to persist as it minimises the energy of the system. This effect retards dislocation motion and a greater shear stress is required to move the dislocation from this configuration than was necessary to move through the host lattice, which gives rise to solute solution strengthening.

