Zinc/carbon batteries
This is commonly known as the Leclanché Cell and despite being the oldest type of primary battery it is still the most commonly used as it is very low-cost.

Georges Leclanché
The first cell was produced by Georges Leclanché in 1866 and was the first cell to contain only one low-corrosive fluid electrolyte with a solid cathode. This gave it a low self discharge in comparison to previously attempted batteries. The original cell consisted of a solid Zinc anode with an ammonium chloride solution as the electrolyte immobilized in the form of a paste (hence called a “dry cell”), and an 1:1 mixture of powdered carbon and manganese dioxide packed around a carbon rod acting as a cathode. In another version for extra heavy-duty applications, the electrolyte is zinc chloride mixed with a small amount of ammonium chloride. The most common variant is the alkaline cell where the electrolyte is potassium hydroxide.
![]()
Characteristics in brief
Voltage: 1.5 – 1.75 V
Discharge characteristics: Generally sensitive to external factors. Generally very sloped. Better when discharged intermittently.
Service Life: 110 min (continuous use)
Shelf life: ~ 1 – 2 years (at room temperature)
![]()
Chemistry
The zinc/carbon cell uses a zinc anode and a manganese dioxide cathode; the carbon is added to the cathode to increase conductivity and retain moisture; it is the manganese dioxide that takes part in the reaction, not the carbon.
The overall reaction in the cell is:
Zn + 2MnO2 → ZnO + Mn2O3
The exact mechanism for this is complicated, and there is still controversy over the exact mechanism, however the approximate half-cell reactions are:
Anode: Zn → Zn2+ + 2e–
Cathode: 2NH4+ + 2MnO2 + 2e– → Mn2O3 + H2O + 2NH3
However, this is complicated by the fact that the ammonium ion produces 2 gaseous products:
2NH4+ + 2e– → 2NH3 + H2
These products must be absorbed in order to prevent build up of pressure in the vessel. This occurs by 2 mechanisms:
ZnCl2 + 2NH3 → Zn(NH3)2Cl2
2MnO2 + H2 → Mn2O3 + H2O
![]()
Construction
The cell has two basic designs: the cylindrical cell and the flat cell.
Cylindrical Cell
The zinc serves as both the container and the anode. The manganese dioxide/carbon mixture is wetted with electrolyte and shaped into a cylinder with a small hollow in the centre. A carbon rod is inserted into the centre, which serves as a current collector. It is also porous to allow gases to escape, and provides structural support. The separator is either cereal paste or treated absorbent kraft paper (the kind of brown paper used to make large envelopes or grocery bags).
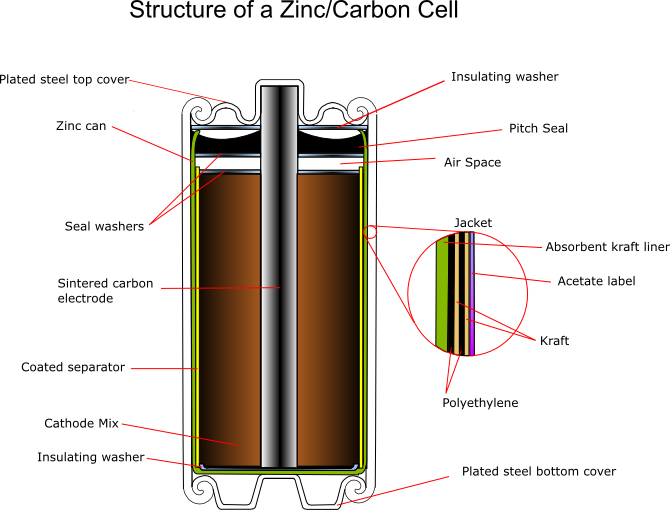
Carbon cathode
This is made of powdered carbon black and electrolyte. It adds conductivity and holds the electrolyte. The MnO2 to Carbon ratios vary between 10:1 and 3:1, with a 1:1 mixture being used for photoflash batteries, as this gives a better performance for intermittent use with high bursts of current. Historically the carbon black was graphite, however acetylene black is often used in modern batteries as it can hold more electrolyte.
Manganese dioxide
everal grades of MnO2 are available:
- Natural Manganese Dioxide: ores occur naturally in Gabon, Greece and Mexico with 70 – 85% MnO2
- Activated Manganese Dioxide
- Chemically synthetic Manganese Dioxide: 90 – 95% MnO2
- Electrolytic Manganese Dioxide (EMD): higher cell capacity and rate capabilities, and less polarization. Used in industrial applications.
Electrolyte
A standard Leclanché cell uses a mixture of ammonium chloride and zinc chloride in aqueous solution. A zinc-corrosion inhibitor is also added, which forms an oxide layer. This inhibitor is usually mercuric oxide or mercurous chloride.
A typical electrolyte composition is:
| NH4Cl | 26.0% |
| ZnCl2 | 8.8 % |
| H2O | 65.2 % |
| Corrosion inhibitor | 0.25 - 1.0 % |
Carbon rod
This is inserted into the cathode and acts as a current collector. It also provides structural support and vents hydrogen gas that evolves as the reactions proceed. When raw the rods are very porous, so must be treated with waxes or oils to prevent loss of water, but remain porous enough to allow hydrogen to pass through. Ideally they should also prevent oxygen entering the cell, as this would aid corrosion of the zinc.
Separator
This can be either a gelled paste, or kraft paper coated with cereal. It physically separates the anode and the cathode, but allows ionic conduction to occur in the electrolyte.
Paste: The paste is flowed into the zinc can, and the carbon cathode inserted, forcing the paste up the sides of the can between the zinc and the cathode, where it sets.
Paper: The paper is coated with cereal, or another gelling agent, rolled into a cylinder and along with a circular bottom sheet is added to the can. The carbon cathode is added, and the rod inserted, pushing the paper against the walls of the cans. This compression releases some electrolyte from the cathode mix, soaking the paper.
As the paste is relatively thick, more electrolyte can be held by the paper than the paste, giving increased capacity, thus paper is usually the preferred separator.
Seal
This can be asphalt pitch, wax/resin mix, or plastic (usually polyethylene or polypropylene) An airspace is usually left between the seal and the cathode to allow for expansion. The function of the seal is to prevent evaporation of the electrolyte, and to prevent oxygen entering the cell and corroding the zinc.
Jacket
The jacket provides strength and protection, and will hold the manufacturers label. It contains various components, which can be metal, paper, plastic, mylar, cardboard (sometimes asphalt-lined) and foil.
Electrical contacts
These are the terminals of the battery, and are tin plated steel or brass. They aid conductivity and prevent exposure of the zinc.

