Electrocatalysis
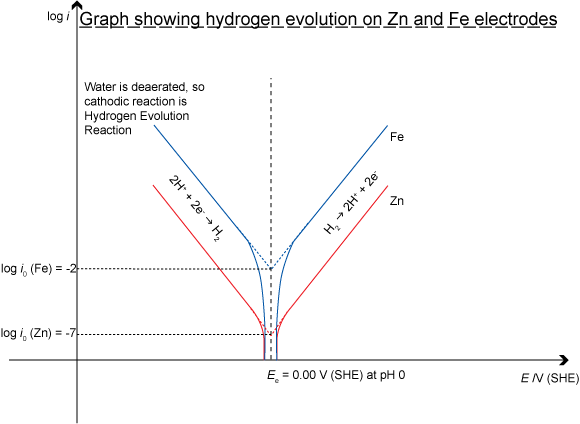
The hydrogen evolution reaction proceeds faster (has a higher i0 value) on an iron surface than on a zinc surface. Iron is said to be a better electrocatalyst of the hydrogen evolution reaction than zinc. The best electrocatalyst of all for the H2 evolution reaction is platinum, which is one of the reasons it is used for the electrodes in fuel cells that utilise the H2 evolution reaction.
In the following graph, data was taken in an experiment where samples were reacted with hydrochloric acid. One sample was pure zinc granules, the other was zinc granules with copper granules added so the surface are of the two samples was roughly constant:
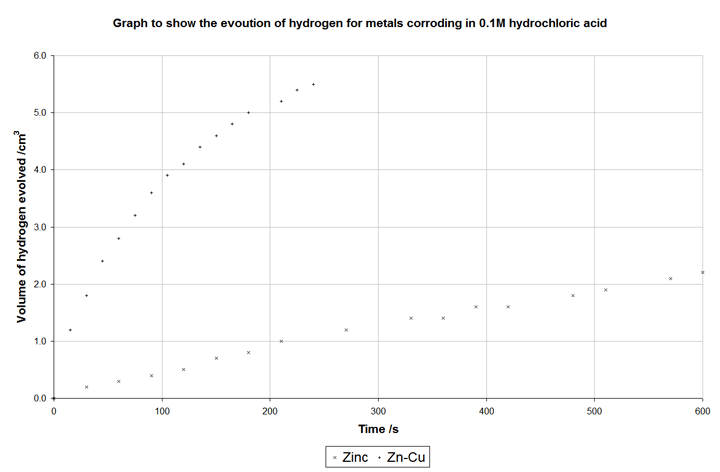
The copper does not corrode, but it does act as the site of the cathodic (hydrogen evolution) reaction. It is a better electrocatalyst than zinc, meaning that the hydrogen evolution reaction proceeds much faster than it does on zinc and, consequently, corrosion is allowed to occur faster – in fact ten times faster than without the copper.
It is easy to see the effect on Tafel plots of different exchange current densities – a measure of the electrocatalytic ability of a material. If the Tafel plots for the corrosion of zinc and the corrosion of iron are plotted on the same axes: