Questions
Quick questions
You should be able to answer these questions without too much difficulty after studying this TLP. If not, then you should go through it again!
-
What might cause a material not to form the phases predicted in the phase diagram after solidification?
-
Which assumptions must you make for the Scheil equation to be valid? (You may select more than one answer)
-
When solidifying a hypoeutectic alloy, during which stage of solidification might you form solid with a eutecticstructure?
-
Which of the following reduce the likelihood of dendrites forming in a binary alloy? (You may select more than one answer)
Deeper questions
The following questions require some thought and reaching the answer may require you to think beyond the contents of this TLP.
-
A bar of magnesium is contaminated with aluminium, at a concentration of 2 wt%. The maximum acceptable concentration is 1 wt%. It is to be purified by directional solidification with a planar growth front, and complete mixing in the liquid. Use the Al-Mg interactive phase diagram to estimate the fraction of the bar that will have an acceptable purity.
-
How would the solute profile of a bar, solidified with complete mixing in the liquid, be affected if some diffusion could occur in the solid?
-
An Al-5wt%Cu alloy undergoes steady state solidification, with a growth front velocity of 10 μm s-1. The diffusivity in the liquid is 10-8 m2 s-1. The equipment being used is capable of applying a temperature gradient of up to 104 K m-1, is it possible to ensure that the alloy will solidify with a planar growth front?
- Use the equation:
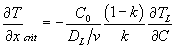 to determine the critical temperature gradient in the liquid.
to determine the critical temperature gradient in the liquid. - The partition coefficient, k, and the liquidus gradient,
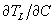 , can be calculated from the phase diagram. (You will need to approximate the liquidus as a straight line.)
, can be calculated from the phase diagram. (You will need to approximate the liquidus as a straight line.)
- Use the equation:
Open-ended questions
The following questions are not provided with answers, but intended to provide food for thought and points for further discussion with other students and teachers.
-
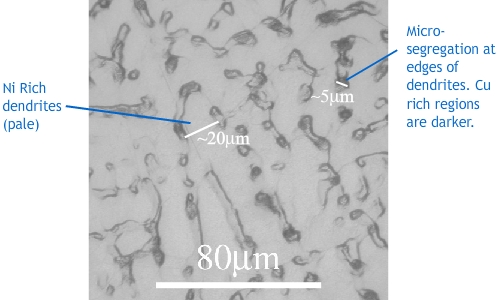
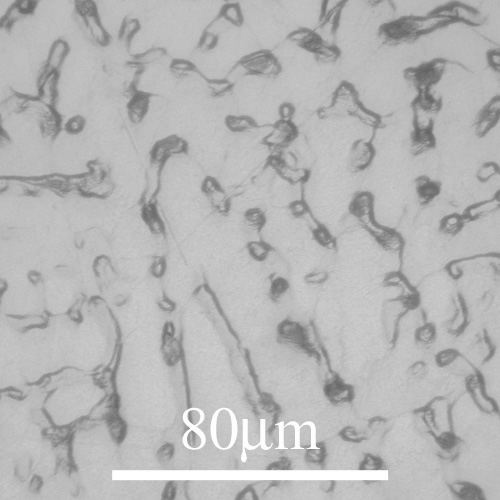
The micrograph shows a Cu-30wt%Ni alloy that has been chill cast (cast in a mould that is kept much colder than the fusion temperature of the alloy).
Look at the phase diagram, and rationalise the features present in the sample. What is the approximate length scale of the microsegregation in the sample?
The grain structure is dendritic, with primary arm widths of ~20 mm. The formation of dendrites has been caused by constitutional undercooling, which would be unavoidable in chill casting because the high cooling rate means that the growth front velocity is very high.
There is microsegregation, with nickel rich regions showing up pale, and regions lower in nickel appearing darker. The phase diagram shows that the system has a partition coefficient greater than one, so the first solid formed is nickel rich (more impurity), and the final solid to form, between the dendrites is low in nickel. The length scale of the microsegregation is approximately 5 mm.