Thermally induced recovery of the shape of a spring (all content)
Note: DoITPoMS Teaching and Learning Packages are intended to be used interactively at a computer! This print-friendly version of the LDP is provided for convenience, but does not display all the content of the LDP. For example, any video clips and answers to questions are missing. The formatting (page breaks, etc) of the printed version is unpredictable and highly dependent on your browser.
Contents
Introduction
This LDP concerns the shape memory effect. This is a relatively complex effect, which is associated with shape changes being effected via martensitic (shear) phase transformations. Since they occur via the systematic, simultaneous displacement of large numbers of atoms, all of which retain the same neighbouring atoms (albeit in a slightly different configuration), there is potential for such shape changes to be reversed, provided the phase change can be reversed. This is most commonly achieved via a change in temperature, which affects the relative thermodynamic stability of the two phases. The actual demonstration, involving plastic deformation of a spring, which is subsequently reversed by simply heating with a hair dryer, is quick and easy to carry out. It's probably worthwhile, however, to also show some illustrations of the phase changes on an atomic scale. An IT resource is included in this package for exploring the crystal structures involved in the actual alloy used in the demonstration, which is Ni-50Ti. A brief explanation is also included of the "training" process that defines the "preferred" shape of the specimen.
It may be noted that there is a TLP focussed on the shape memory effect, accessible at Superelasticity and Shape Memory Alloys. It may be helpful to have a look at this resource beforehand.
Shape changes arising from dislocation glide and deformation twinning (a shear transformation)
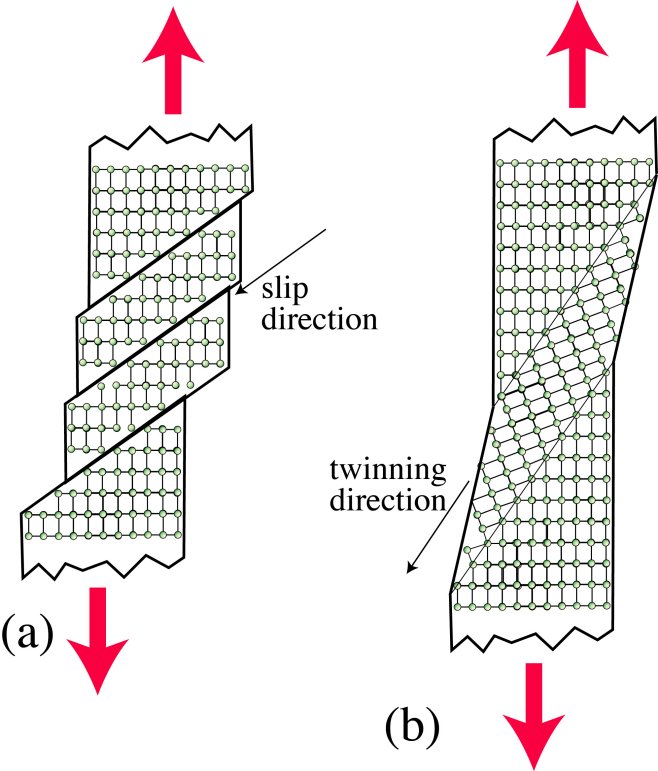
The figure schematically depicts the motion of atoms during (a) dislocation glide and (b) deformation twinning, stimulated in both cases by an applied tensile stress. Slip tends to take place by repeated passage of dislocations on specific slip planes. For a single crystal, in the early stages (as depicted here), the slip takes place on a single system (slip plane and slip direction), with little or no interference between individual dislocations. The schematic does not show individual dislocations, but illustrates the displacement of parts of the lattice, relative to other parts, created by their passage. For deformation twinning, on the other hand, the simultaneous, rapid displacement of a large number of neighbouring atoms, all in the same direction, also creates a (shear) displacement of the lattice, although in this case the sheared region has a different orientation from that of the parent crystal. In fact, the structure is a mirror image of the parent, reflected across the twin plane.
Sequence of microstructural changes during the shape memory effect
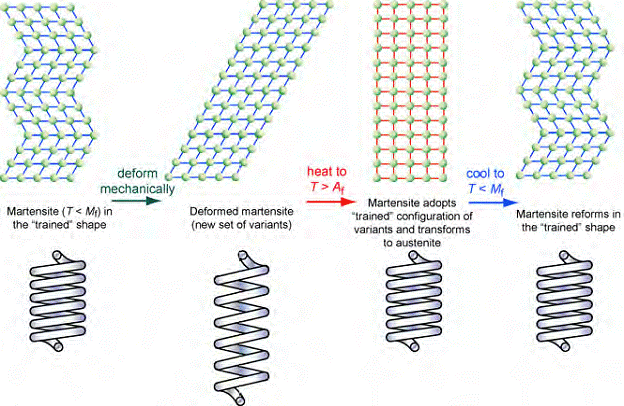
This figure shows schematically what happens inside the material during the demonstration. The material is initially in the martensitic form and the applied "plastic" deformation induces straining in the form of (shear) reorientation of small regions (to form different "variants"). When the specimen is heated, the austenitic structure becomes the one that is thermodynamically more stable. The transformation to austenite takes place in the "trained" shape - the coiled spring in this case. Final cooling leads to the crystal structure reverting to martensite, while the shape remains the same. "Training" of this sample involved heating and then fairly rapid cooling while it was constrained into the shape of the coil. This created a set of residual stresses that ensure that the phase transformation takes place most readily when the specimen has this shape.
IT resources - crystallography of the phase transformations in the Ni-50Ti alloy
This animation allows the crystal structures adopted by the Ni-Ti alloy over the temperature range concerned to be viewed and rotated. The sequence of phase formation during heating and cooling is outlined within the animation, and is also presented in the video of the demonstration.
Equipment and facilities needed for the demonstration

Lecture demonstration equipment
The equipment required is very simple - essentially just a standard hair dryer and the coil. It would also be helpful to have a small tray in which to retain the coil while it is being heated. There is also the issue of visibility. Unless the audience is very small, and can look down on the tray, they are unlikely to be able to see clearly what is happening. The best solution to this is to have a visualiser (camera sending its signal to a projector) available. Alternatively, an overhead projector can be used, although the visibility of the coil is unlikely to be as good as with a visualiser. Of course, it's also possible to just show a video of the demonstration, although there's no doubt that there are several advantages to having a "real" demonstration.
The key challenge is clearly that of obtaining a suitable coil. Actually, this is relatively easy. Wire of Ni-Ti (Nitinol) is obtainable commercially, and is not usually very expensive. It's probably best, if possible, to buy it as conventional wire - ie not trained into any shape - and to carry out the "training" in house. This will certainly be cheaper than buying a "trained" component. The training consists of constraining it into the shape concerned, heating it (to about 400-500°C) holding it for a few minutes and then quenching it to room temperature, probably just by removing it from the furnace and allowing it to cool in air. (Some trial and error may be needed, since the details depend on several factors, notably the composition and the diameter of the wire.) Various shapes could be produced, although a coiled spring is obviously an easy one. There are naturally some health and safety issues during the training operation - ie heating to ~500°C, and then cooling fairly quickly, but there are none of any significance during the demonstration itself.
Video of the demonstration
Academic consultant: Bill Clyne (University of Cambridge)
Content development: Nadia Shatrova
Photography and video: Steve Penney, Jess Gwynne
Web development: Lianne Sallows and David Brook
This DoITPoMS LDP was funded by the UK Centre for Materials Education and the Department of Materials Science and Metallurgy, University of Cambridge.

