Hexagonal close-packed crystals: the axial ratio
The ideal axial ratio c/a for a hexagonal close-packed crystal structure can be calculated by considering non-interacting identical hard spheres packed together in the h.c.p. crystal structure.
If the sphere radius is r, then the lattice parameters a (=b) and c can be written in terms of r:
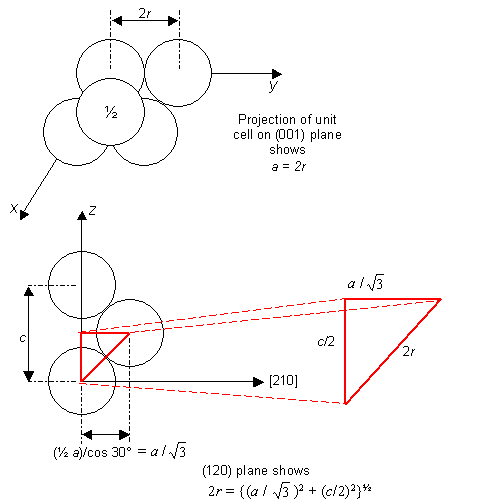
These two relationships can be solved for the ideal axial ratio c/a:
$$2r = a = {\left( {{{{a^2}} \over 3} + {{{c^2}} \over 4}} \right)^{1/2}}$$
a2 = a2/3 + c2/4
4 = 4/3 + c2/a2
c/a = 1.633
Many metallic materials have the h.c.p. crystal structure, but the axial ratio is rarely ideal. Cadmium, for example, has an axial ratio of c/a = 1.886. This non-ideal structure has implications for the behaviour of the material, for example in slip.

