Applications
Silicon chips
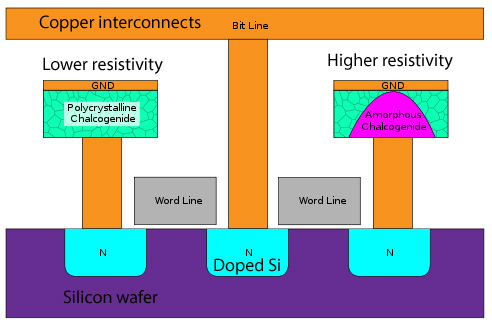
As electrical properties vary with microstructure, a type of computer memory called phase-change random-access memory (PC-RAM) has been developed. The material used is a chalcogenide reffered to as GST (Ge2Sb2Te5).
The amorphous state is semiconducting, while in a (poly)crystalline form it is metallic. Heating above the glass transition, but below the melting point, crystallises a previously semiconducting amorphous cell. Likewise, fully melting, then rapidly cooling a cell leaves it in the metallic crystalline state.
This variation of resistivity with microstructure is crucial to the operation of such devices. By varying the heating conditions, a varying proportion of each GST cell may be crystalline and amorphous - the mixture rule applies as it’s effectively two phases. This allows for multiple distinguishable levels of resistance per cell, increasing the storage density, and reducing the cost per megabyte.
The more common problem with silicon devices is dissipating heat.
A modern processor has a thermal design power of above 70w (Intel i7 3770, 22 nm process). A cooler must dissipate that specified amount of heat from the die’s surface, which is typically less than 10 cm2. It is common for heat sinks to have a copper block attached to the microprocessor casing by thermal paste, and pressure. The bulk of the heat sink is usually made from much cheaper aluminium, though the high thermal conductivity of copper is necessary for the interface. Thermal paste, whilst a better thermal conductor than air, is much worse than most metals, so it is only used as a thin layer to replace air gaps.
Conduction is not the most efficient method to carry heat to a separate heat sink, so convection and the latent heat of evaporation can be used. Heat pipes, typically made from copper are filled with a low boiling point liquid, which boils at the hot end, and condenses at the cool end of the pipe. This is a much faster way of transferring heat over longer distances.
Space
There are many applications of thermal insulators, with development coming from attempts to improve bulk mechanical properties, while retaining insulating properties, (i.e. Don’t let heat through, but don’t melt)
A particularly famous application of thermal insulation is the (now retired) space shuttle tiles which are responsible for protecting the shuttle during re-entry into the atmosphere. They are such good insulators, that the outside may glow red-hot, while inside the shuttle the astronauts are still alive.
One of the best thermal insulators is silica aerogel.
An aerogel is an extremely low-density solid-state material made from a gel where the liquid phase of the gel has been replaced with gas. The result is an extremely low density solid, which makes it effective as a thermal insulator.
One use of aerogels is for a lightweight micrometeorite collector, aerogel was used. While extremely light, it is strong enough to capture micrometeors.
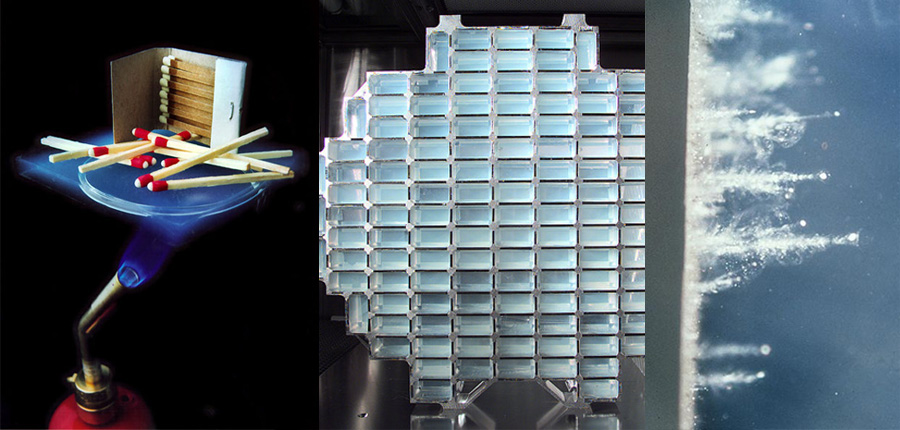
Matches stay cool millimetres from a blowtorch, a large array of aerogel bricks is ready to be launched into space, and the resulting space dust is photographed upon return to earth
Aerogels can be made from a variety of materials, but share a universal structure style. (amorphous, open-celled “nanofoams”). However, a common material used is silicate. Silica aerogels were first discovered in 1931.
Aerogels have extreme structures and extreme physical properties. The highly porous nature of an aerogel structure provides a low density. The percentage of open space within an aerogel structure is about 94% for a gel with a density of 100 kg m-3.
Aerogels are good thermal insulators because they eliminate the three methods of heat transfer (convection, conduction and radiation). They are good convective insulators due to the fact that air cannot circulate throughout the lattice. Silica aerogel is an especially good conductive insulator because silica is a poor conductor of heat - a metallic aerogel, on the other hand, would be a less effective insulator. Carbon aerogel is an effective radiative insulator because carbon is able to absorb the infrared radiation that transfers heat. Hence, for maximum thermal insulation, the best aerogel is silica doped with carbon.
Power transmission
One of the largest scale uses of electrical conductors is in power transmission.
Unfortunately, the properties that are desirable for a strong cable seem opposed to those for a good conductor.
Aluminium alloys can be very strong for their density, but following Nordheim’s rule, are much poorer conductors.
There are a huge variety of steels, but again, the interstitial carbon atoms increase the resistance compared to pure iron. This means that a larger diameter cable is needed, which, due to the density of steel, ends up being very heavy and expensive. Heavier cable also means we must construct additional pylons, which is a large component of the cost.
Copper, while appropriate for home wiring, is dense, and increasingly expensive.
For most overhead power cables, the solution is to use two materials – a steel core, surrounded by many individual aluminium cores. This achieves light, high strength, and acceptable conductivity cables.
Superconductors have been trialled for power transmission, though only underground, and at a considerably higher cost (and efficiency!).
Thermoelectric effect
The thermoelectric effect is the direct conversion of a difference in temperature into electric voltage and vice versa. Simply put, a thermoelectric device creates a voltage when there is a different temperature on each side of the device. It can also be run “backwards”, so when a voltage is applied across it, a temperature difference is created. This effect can be used to generate electricity, to measure temperature, to cool objects, or to heat them. Because the sign of the applied voltage determines the direction of heating and cooling, thermoelectric devices make very convenient temperature controllers.
The Peltier effect is that when a (direct) current flows through a metal-semiconductor junction, and heat is either absorbed or released. This is because the average energy of electrons in the two materials is different, and heat makes up this difference.
A fuller understanding requires knowledge of the band structure, explored further in the TLP on Semiconductors.

