Diffusion mechanisms
Diffusion can occur by two different mechanisms: interstitial diffusion and substitutional diffusion. Picture an impurity atom in an otherwise perfect structure. The atom can sit either on the lattice itself, substituted for one of the atoms of the bulk material, or if it is small enough, it can sit in an interstice (interstitial). These two positions give rise to the two different diffusion mechanisms.
Substitutional Diffusion
Substitutional diffusion occurs by the movement of atoms from one atomic site to another. In a perfect lattice, this would require the atoms to “swap places” within the lattice. A straight-forward swapping of atoms would require a great deal of energy, as the swapping atoms would need to physically push other atoms out of the way in order to swap places. In practice, therefore, this is not the mechanism by which substitutional diffusion occurs. Another theoretical substitution mechanism is ring substitution. This involves several atoms in the lattice simultaneously changing places with each other. Like the direct swap method, ring substitution is not observed in practice. The movement of the atoms required is too improbable.
Substitutional diffusion occurs only if a vacancy is present. A vacancy is a “missing atom” in the lattice. If a vacancy is present, one of the adjacent atoms can move into the vacancy, creating a vacancy on the site that the atom has just left. In the same way that there is an equal probability of an atom moving into any adjacent atomic site, there is an equal probability that any of the adjacent atoms will move into the vacancy. It is often useful to think of this mechanism as the diffusion of vacancies, rather than the diffusion of atoms.
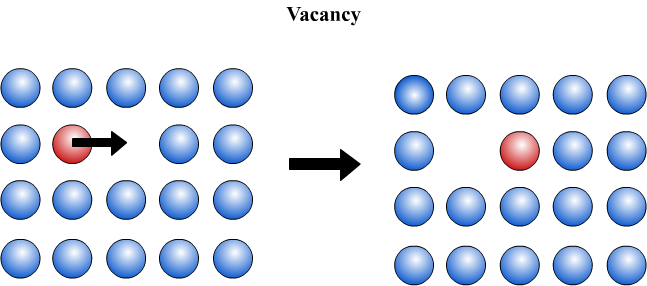
The diffusion of an atom is therefore dependent upon the presence of a vacancy on an adjacent site, and the rate of diffusion is therefore dependent upon two factors: how easily vacancies can form in the lattice, and how easy it is for an atom to move into a vacancy. The dependence upon the presence of vacancies makes substitutional diffusion slower than interstitial diffusion, which we will look at now.
Interstitial Diffusion
In this case, the diffusing atom is not on a lattice site but on an interstice. The diffusing atom is free to move to any adjacent interstice, unless it is already occupied. The rate of diffusion is therefore controlled only by the ease with which a diffusing atom can move into an interstice. Theoretically, at very high impurity concentrations movement may be restricted by the presence of atoms in the adjacent interstices. In practice, however, it is very likely that a new phase would be formed before this had an effect.

