Fick's first law
We have looked at the mechanisms of on an atomic scale. We now want to examine the emergent properties of these mechanisms when there are a lot of atoms. The first thing to note before we start is that no real material has a perfect structure. There will some amount of vacancies or other imperfections present. Therefore if we add impurity atoms to the material, they will be able to move around the material at some rate. If they are interstitial they will move around at a faster rate since they do not require any vacancies to move.
The second thing to note is that if the impurity atoms are distributed evenly throughout the material such that there is no concentration gradient, their random motion will not change the concentration of the material. Nor will there be a net movement of atoms through the material. We are interested in the cases where there is some kind of energy difference in the material, which causes a net movement of atoms. This can be caused by concentration differences, electric fields, chemical potential differences etc.
We will first look at the case of concentration differences. The fact that a concentration difference causes diffusion should be familiar to everyone, particularly in the case of liquids and gases. Consider adding a drop of ink to a bowl of water. The ink will diffuse through the water until the concentration is the same everywhere. There is no force causing the ink particles to diffuse through the water. It is in fact a statistical result of the random motion of the particles.
We can use Fick’s laws to quantitatively examine how the concentrations in a material change.
Consider a crystal lattice with a lattice parameter λ, containing a number of impurity atoms. The concentration of impurity atoms, C (atoms m-3) may not be constant over the whole crystal. In this case there will be a concentration gradient across the crystal, which will act as a driving force for the diffusion of the impurity atoms down the concentration gradient (i.e. from the area of high concentration to the area of low concentration).
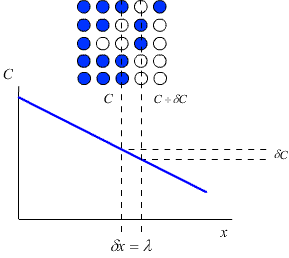
Fick’s first law relates this concentration gradient to the flux, J, of atoms within the crystal (that is, the number of atoms passing through unit area in unit time)
Fick’s first law (derivation here) is \(J \equiv - D\left\{ {\frac{{\partial C}}{{\partial x}}} \right\}\)
D is the diffusivity of the diffusing species.
Our equation relating the mean diffusion distance to time can now be modified to be in terms of this parameter:
\[\begin{array}{l} \overline x = \lambda \sqrt {\nu t} \\ D = \frac{1}{6}\nu {\lambda ^2}\\ \overline x = \sqrt {6Dt} \\ \overline x \approx \sqrt {Dt} \end{array}\]
The animation below demonstrates Fick’s 1st Law with respect to a fluid.

