Questions
Quick questions
You should be able to answer these questions without too much difficulty after studying this TLP. If not, then you should go through it again!
-
What is the effect on the shape of the free-energy curve for a solution if its interaction parameter is positive?
-
In terms of interatomic bonding, what does a negative interaction parameter represent?
-
Cooling curve for a binary system:
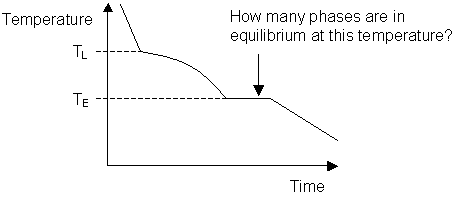
-
What is a hypoeutectic alloy?
-
Look at the following Al-Si phase diagram. (Place the mouse over the diagram to determine the temperature and composition at any point.)
For an Al 5 wt% Si alloy what will be the composition of the solid in equilibrium with the liquid at 600°C?
-
Look at the following Cu-Al phase diagram. (Place the mouse over the diagram to determine the temperature and composition at any point.)
What will be the relative weight fraction of CuAl2 (θ) in a Al 15wt% Cu alloy at its eutectic temperature?
Deeper questions
The following questions require some thought and reaching the answer may require you to think beyond the contents of this TLP.
-
Using the following data, calculate the volume fraction of the beta phase and eutectic at the eutectic temperature, for an alloy of composition 75 wt% Ag. Assume equilibrium conditions.
At eutectic temp:
eutectic composition = 71.9 wt% Ag
maximum solid solubility of Cu in Ag = 8.8 wt% Cu
density Ag = 10 490 kg/m3
density Cu = 8 920 kg/m3 -
Composition vs temperature phase diagrams exist for the combinations of three elements A, B and C (i.e. the three phase diagrams A-B, A-C and B-C). How might they be arranged in three-dimensional space to construct a "ternary" phase diagram for a system containing A, B and C?
Open-ended questions
The following questions are not provided with answers, but intended to provide food for thought and points for further discussion with other students and teachers.
-
Under what conditions could the compositions of the phases present differ from that predicted in the phase diagram?

