• System and outlook
The operating temperature of an SOFC is relatively high.
A typical SOFC power plant is fuelled with natural gas because of the lack of a hydrogen infrastructure. A plant must have three main components:
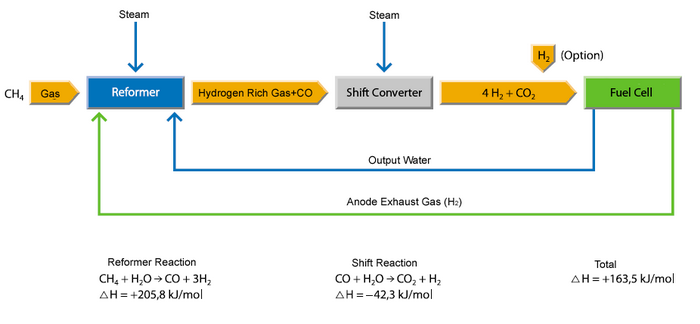
1. The preheater raises the temperature of the fuel and air to near the operating temperature. At the same time, the preaheater reforms the gas by steam reforming to hydrogen. Steam reforming constitutes of two steps:
Methane Reforming: CH4 + H2O → CO + 3H2 |
2. The cell stack electrochemically oxidises the hydrogen stream, drawing oxide ions through the electrolyte from the air stream.
Electrochemical reaction: H2 + ½O2 → H2O |
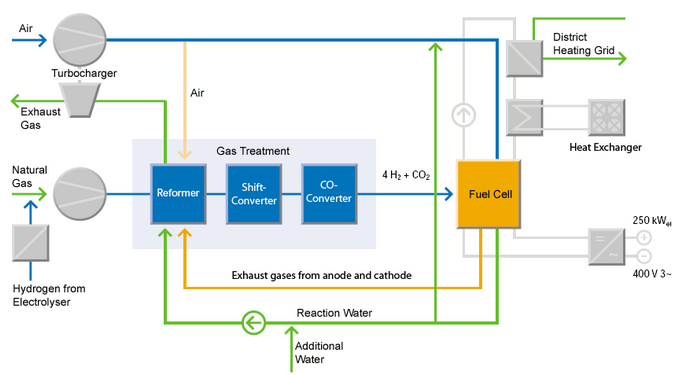
The schematic diagram above depicts a complete, 250 kW fuel cell system (Source: Innovationspark-Brennstoffzelle).
3. The lower cycle utilises the exhaust energy. The exhaust gases are so hot that gas turbines can be driven to generate additional electrical energy and thus increasing the efficiency of the fuel cell system up to 80%.

SFC-200, a 125 kW SOFC cogeneration system (Source: Siemens Westinghouse)
The durability of the SOFC is mainly determined by the processes occurring during thermal cycles, oxidation-reduction cycles and the sulphur contamination (even at high temperatures, sulphur is absorbed by the anode).
Fuel
One of the great advantages of the SOFC is that it can use a big range of fuels, depending on the cathode composition. Due to the high operating temperature, internal reforming can take place at the anode, when steam is added to the fuel. The reaction of methane is as follows:
CH4 + H2O → CO + 3H2 |
Both hydrogen and carbon monoxide can react with the oxide ions. A shift reaction also occurs at the anode since the reaction of CO is slow, producing more hydrogen.
CO + H2O → CO2 + H2 |
The disadvantage of using hydrocarbon fuels is the possible formation of coke on the anode:
2CO → CO2 + C |
As mentioned above, impurities, such as sulphur are also damaging to the SOFC. Only desulphurised natural gas can be used as fuel. Other additives (more than 100 different molecules are present in commercial gasoline) can have damaging effects on the nickel anode.
The activity of the nickel anode decreases due to sintering and coke formation when carbon containing fuels are used. The ceramic parts can easily break if vibrational forces are present. This is one reason, why SOFCs are best suited for stationary applications rather than mobile applications.
The ultimate goal is to build a decentralised network of medium sized power generating SOFCs that can supply a small community with electricity with a much higher reliability and minor consequences in case of failure compared to the current system of few but very large power plants.

