Theory of rubber conformation
Polymer Coils
Polymer molecules are made up of many smaller units called monomers. A rubber is a fully amorphous, lightly cross-linked polymer, above Tg. They are normally composed of a -C-C- backbone chain. The bond angle is fixed at 109.5°, but the torsion angle can change, allowing the macroscopic shape of the chain to vary from being linear to being highly coiled and convoluted.
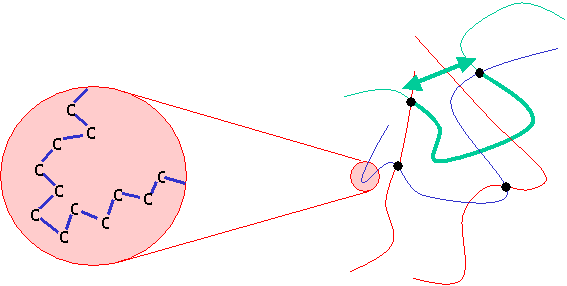
In this diagram, on the left each blue line represents a C-C link. The arrow shows the end-to-end distance of the chain segment, depicted as a thickened line. The segments tend to coil up to some extent, rather than aligning in a straight line. This can be thought of as the system increasing its entropy. The probability distribution for the end-to-end distance can be described mathematically by a Gaussian function:
\[P\left( {{{\vec r}_1},........,{{\vec r}_N}} \right) = {\left\{ {\frac{3}{{2\pi {b^2}}}} \right\}^{3N/2}}\exp \left\{ { - \sum\limits_{i = 1}^N {\frac{3}{{2{b^2}}}r_i^2} } \right\}\]
Tangles and Crosslinks
A piece of rubber, such as a rubber ball or a rubber band, is made up of many polymer molecules. As the molecules prefer to be coiled to a certain degree, rather than stretched out, the polymer molecules easily get tangled together. When chains become entangled, their mobility decreases. Furthermore, the entanglements mean that the chains cannot stretch as far as otherwise they would be able to and so the stiffness of the rubber increases - at least if it is measured over short timescales, which do not permit the entanglements to slide.
As well as physical entanglements, the chains can join together in another manner. If the chemistry of the chain is suitable, an atom belonging to one chain can form a chemical bond with an atom from another chain. This bond is called a cross-link. The nature of the cross-linking bonds is covalent. The cross-links inhibit the motion of the polymer chains and so increase the stiffness of the rubber. These are now stable over long time scales, so the stiffness is not time-dependent.
Coiling and Uncoiling
Consider what happens when you stretch a rubber band or a balloon. We know that the rubber will stretch a long way before it breaks, but we ought to be able to explain why it behaves the way it does.
When you first put the rubber under tension, the polymer molecules will begin to change their conformation. Pulling on the chains makes the polymers uncoil. This is shown schematically below:
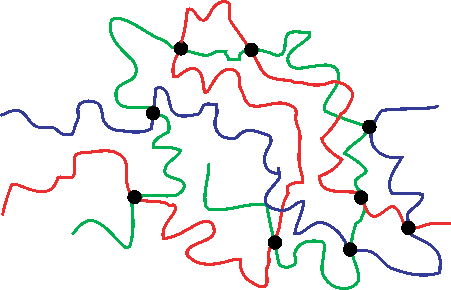
Unloaded coiled chains
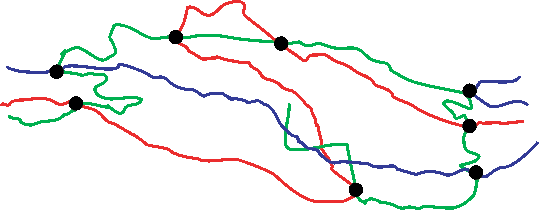
Loaded in tension
As you continue to pull on the rubber, the chain segments start to reach their limits of extensibility. In the case of silly putty or chewing gum, this sliding can continue until the chains no longer make contact and the rubber gets drawn out to a very thin cross section and perhaps fractures.
For conventional cross-linked rubbers, on the other hand, the chain segments uncoil as far as they can before the cross-links inhibit further uncoiling. Further tension now pulls directly on the C-C bonds of the polymer backbone. When the force becomes great enough, the C-C bonds will break and the rubber will snap. The strength of the rubber is thus not very different from other materials, whereas the stiffness is lower by orders of magnitude.
We should now be able to predict the shape of the extension vs force graph when extending rubber. This will be done for both uniaxial and biaxial tension later in the TLP.
Effect of sun on rubber band
Have you ever noticed what happens to a rubber band when it is left out in the sunshine for too long? The rubber becomes brittle and can break in your hand. The explanation for why this happens concerns cross-linking bonds.
Ultra-violet light from the sun provides the polymer molecules with the activation energy they need to be able to form more cross-links with other chains. When the rubber band is left out for a long time, the density of cross-links increases. When you try and stretch the rubber band, the chains are prevented from uncoiling or sliding past each other, due to the large number of cross-links. Because of this you are effectively pulling on the C-C backbone bonds of the polymer, which are very stiff and will not stretch much. Instead the rubber band snaps with very little extension.
Some oils and other chemicals have a similar effect on rubbers. However, butyl rubbers have a much lower density of available cross-link sites than other rubbers. Because of this it is much more difficult to form excess cross-link bonds and so butyl rubbers are resistant to degradation from U.V. light and from oils.

