Transformation toughening
Tough materials can absorb energy at or around the crack tip, by processes such as plastic flow in metals. Usually ceramics cannot do this to a significant degree, so fail by brittle fracture. However, some ceramics show a stress-induced martensitic transformation. This occurs in a local region (or zone) around the crack tip where stress is concentrated; the far-field stress is insufficient to cause the transformation. As the crack grows, this zone moves through the material.
This martensitic transformation requires energy to be absorbed in order to overcome the energy barrier associated with forming the new phase. Further, if there is a volume increase from the high temperature (austenite) phase to the martensite phase, the local tensile stress at the crack tip will decrease. Both factors hinder crack progression through the material and therefore increase the toughness, leading to the concept of transformation toughening.
Zirconia is a ceramic that can undergo transformation toughening by using a tetragonal to monoclinic martensitic transformation provided it fits the following conditions:
- At the operating temperature the tetragonal phase is metastable, so the work done is not recoverable
- The energy barrier to transformation is small
- A reasonable strain is associated with the transformation
- Work can be done ahead of the crack tip which leads to increased fracture resistance
- There is a volume increase
- The operating temperature needs to be below the martensitic transformation temperature otherwise no toughening can occur by transformation
Assuming no constraints, i.e., assuming (i) the surface energies of the tetragonal and monoclinic phases are identical, (ii) that the condition for transformation is independent of the size of the region to be transformed, and (iii) that the volumes of the two phases for a given mass are identical, the driving force of the transformation per unit volume (of the tetragonal phase) is:
\[ \Delta {U^{(\text{t} \to \text{m})}} = \Delta G = {G_ \text{m}} - {G_ \text{t}}\]
\[ {\Delta G} = {\Delta H} - {T\Delta S} \]
where \( G_ \text{m} \) and \( G_ \text{t} \) are the Gibbs free energy in joules per unit volume of the monoclinic and tetragonal phase respectively.
We can assume that enthalpy does not vary significantly with temperature; this is a very reasonable approximation. The equilibrium temperature will be when \( \Delta G = 0 \) because we can ignore any barriers to the phase transformation such as the surface energy. The graph below shows how the driving force changes with temperature.
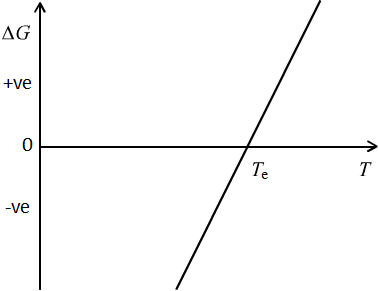
Figure 2: Gibbs free energy per unit volume as a function of temperature
Above the equilibrium temperature, \( {T_ \text{e}} \), \( \Delta G \) is positive because the tetragonal phase is the stable phase. Below the equilibrium temperature \( \Delta G \) is negative because the monoclinic phase is the stable phase. The gradient of the line, \( -\Delta S \), is therefore positive, and so \( \Delta S \) is negative, as is therefore \( \Delta H \), because at \( {T_ \text{e}} \), \( \Delta H = T_ \text{e}\Delta S \). This is a useful check: on cooling through the transformation temperature, \( \Delta H \) will be negative if it is a first order phase transition, consistent with latent heat being released. Hence, \( \Delta S \) is negative.