Making tough zirconia
It is difficult to make zirconia that has a metastable tetragonal phase at room temperature. Very small grains are required because otherwise the grains will spontaneously transform into the stable monoclinic phase.
There is more than one way to make zirconia with a metastable tetragonal phase. The first method is to add alloying additions which lower the cubic solid solution temperature and then follow a particular heat treatment schedule.
One suitable alloying addition is magnesia, MgO. As can be seen in the phase diagram below in Figure 4, the addition of MgO stabilises the cubic solid solution phase to lower temperatures, keeping the tetragonal to monoclinic equilibrium transformation temperature unaffected. The diffusion kinetics of the eutectoid transformation of this cubic phase to tetragonal zirconia and MgO at 1400 °C are very sluggish; this enables the cubic phase to be retained down to room temperature as a metastable matrix phase within which there are fine second phase tetragonal precipitates. The material has the acronym PSZ - partially stabilised zirconia.
The following thermal process can be used to form metastable tetragonal grains:
- Solution treat in the cubic phase field
- Quench to a suitable temperature in the cubic/tetragonal two-phase field
- Age at this temperature to produce tetragonal precipitates. Initially these precipitates form as fine Guinier-Preston (GP) zones.
- Coarsen these GP zones to produce lenticular tetragonal zirconia precipitates only a few nm in size
- Cool to room temperature to prevent the eutectoid decomposition of the cubic zirconia matrix phase
The resulting microstructure is one where the lenticular tetragonal particles are metastable and will not have transformed spontaneously into the stable monoclinic zirconia phase
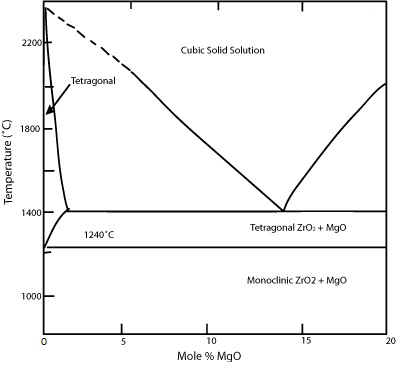
Figure 4: Zirconia-magnesia phase diagram
A second way of making tough zirconia is to add alloying additions which change the transformation temperature. Additions of yttria, Y2O3, and ceria, CeO2, at the level of a few mole per cent are often used to stabilise the tetragonal phase down to room temperature. Yttria-tetragonal zirconia polycrystals (Y-TZP) are commercial ceramics which make use of this ability to stabilise the tetragonal phase down to room temperature.
A third way of making tough zirconia is to increase the elastic resistance to transformation. This can be achieved by having a stiff surrounding matrix within which tetragonal zirconia particles are dispersed (see the equation for \( k \) in the section Effect of constraints). An example of this is zirconia-toughened alumina (ZTA) in which tetragonal zirconia particles are dispersed in a stiff polycrystalline alumina matrix (Figure 5). Alumina is twice as stiff as zirconia. For such ceramics, the matrix must having the following requirements:
- A low solubility for zirconia
- No chemical reactions / intermediate phase formation
- A stiffness higher than zirconia
- It must also be cheaper than zirconia
Other examples of this family of ceramics are mullite-ZrO2, NiAl-ZrO2 and MoSi2-ZrO2.
ZTA is used in applications such as cutting tools for wood and steel where good wear resistance is required, and where very high temperatures are not reached on the surface of the cutting tool.
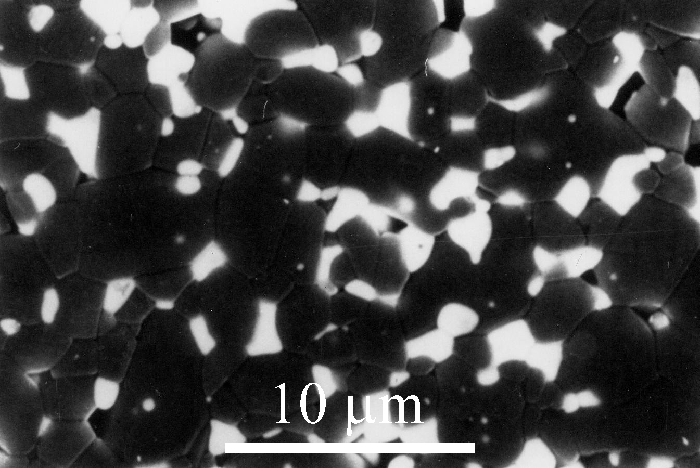
Figure 5: Micrograph 195 from the DoITPoMS library - Micrograph of ZTA

