TRIP and TWIP steels
In addition to dislocation motion, metals may also deform plastically by other mechanisms. One mechanism is a stress-induced transformation of the crystal structure and a second mechanism is stress-induced twinning of the crystal structure. By suitable alloying additions to plain Fe−C steels, these mechanisms can be exploited. These steels are known as TRIP (TRansformation Induced Plasticity) and TWIP (TWinning Induced Plasticity) steels, respectively.
Transformation-induced plasticity steel: TRIP steel
An example of a TRIP steel is Fe with 0.2wt% C, 2wt% Mn and 2wt% Si. This has a ferrite \( \alpha \) matrix with >5% retained austenite. The microstructure also has martensite and bainite in small amounts. As there is a significant volume fraction of retained austenite, it is possible to induce the displacive transformation of the austenite to martensite by straining the steel. The transformation has a large associated transformation strain and allows plastic strains up to 35% to be reached without sacrificing strength.
The relative free energy of the face-centred cubic \( \gamma \) phase (austenite) and the sheared body centred tetragonal martensite phase are shown in Figure 6. On cooling, the austenite does not spontaneously transform when the two free energies are equal (the temperature, \( T_{\text{eq}} \), where the two lines cross) because there are significant energy barriers (strain energy and surface energy both rise when the material transforms). Instead, a significant undercooling is required to cause martensite to form. The proportion of martensite that transforms varies continuously with the cooling below the equilibrium temperature. The onset of martensite formation is denoted \( M_{\text{s}} \), and the end of martensite formation is denoted \( M_{\text{f}} \).
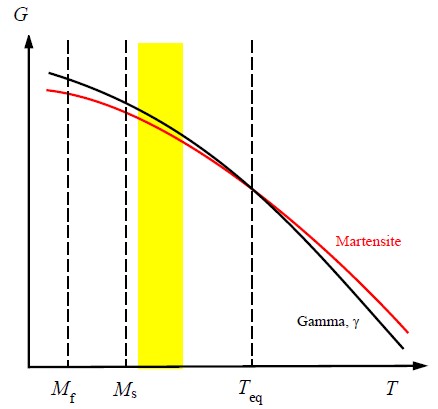
Figure 6: Gibbs free energy, \( G \), of martensite and \( \gamma \) as a function of temperature
In order to exhibit TRIP behaviour, the austenite should be retained, so the service temperature (usually the ambient temperature) must be above \( M_{\text{s}} \), and the barrier to the transformation should not be too large, otherwise the applied stress will not be sufficient to trigger the transformation. Thus, the temperature should be only just above \( M_{\text{s}} \), so that it is within the temperature range defined by the filled yellow rectangle shown in Figure 6. Austenite retained in this way is metastable and if provided with some energy (e.g., by an applied stress) to overcome the barrier, the austenite may transform to martensite. If this occurs, the transformation is irreversible and significant amounts of energy are absorbed.
In addition to the direct work absorbed in inducing the transformation, there is also a contribution to toughness of the material through the relief of locally concentrated stresses, an enhancement of the work hardening rate and allowing deformation to occur homogenously throughout the material to absorb more energy. These effects arise because the strong martensite forms in the regions experiencing the highest strain, locally reinforcing the material, and spreading the deformation to other regions. If the retained austenite is a minor proportion of the microstructure, then the material is sometimes known as TRIP-assisted steel.
TRIP steels, such as Fe−0.2wt% C−2wt% Mn−2wt% Si, are alloyed and heat treated carefully to produce the desired amount of retained austenite and martensite start temperature, \( M_{\text{s}} \), so that toughening is maximised. In particular, the addition of Si suppresses the formation of Fe3C carbides, raising the carbon concentration in the remaining austenite and preventing its transformation to ferrite. Since the austenite phase is stabilised, some will be retained when cooled to room temperature. A TRIP-assisted steel microstructure is shown in Figure 7.
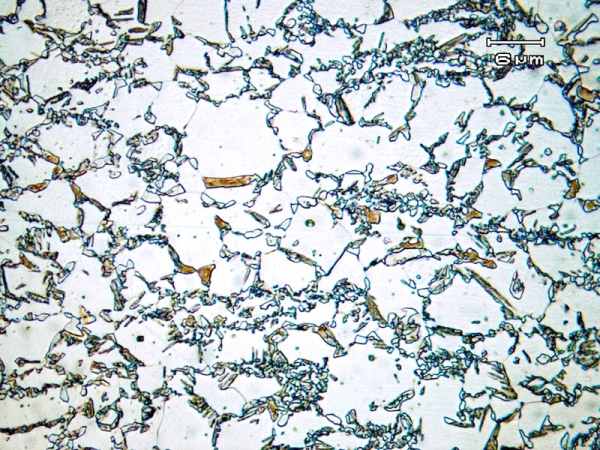
Figure 7: TRIP steel annealed at 775°C for 5 mins and then hold at 400°C for 40s for austenite stabilization The largest grains are ferrite, the darker regions are bainitic, and the smaller white grains are the retained austenite, which will transform to martensite when a stress is applied (micrograph 740 from the micrograph library).
During subsequent deformation, the strain at which the austenite starts to transform is controlled by the carbon content.
If the carbon content is relatively low, the steel is inherently quite formable and the transformation to martensite will occur almost immediately, which increases the work hardening rate because martensite is hard.
If the carbon content is relatively high, the retained austenite is more stable and will only start to transform to martensite when the strain levels are beyond the strains arising during forming operations. This means the material is particularly tough because the transformation that occurs when excessive stress is applied will absorb energy.
As TRIP steel can absorb significant amounts of energy as it gets deformed when excessive stress is applied, it is often used in cars in crumple zones to help absorb energy if there is a collision, and therefore to help protect the car passengers.
Twinning-induced plasticity steel: TWIP steel
TWIP steels are typically made with 15−30 wt% Mn, 3 wt% Si and 3 wt% Al. The reason for such a high Mn content is to ensure the steel has a fully austenitic \( \gamma \) phase microstructure at room temperature.
Mechanical twinning is observed in many materials. This allows the deformation of the material by cooperative shear of a number of planes simultaneously. The result is that potentially very large shear strains are achievable. The distinction between TWIP steels and TRIP steels is that after the shearing of the austenite planes in TWIP steels, the structure is the same austenitic structure, but reoriented relative to the original parent grain.
In TWIP steels this can be exploited by retaining stable austenite to the operating temperature, via the addition of the austenite stabiliser manganese. The addition of some aluminium and silicon reduces the density and helps to control the stacking fault energy to ensure that the material remains as f.c.c. austenite. At high manganese concentrations, the stacking fault energy is sufficiently low that a further TRIP mechanism can occur during deformation, converting the \( \gamma \) phase via a martensitic transformation to the h.c.p. \( \varepsilon \) phase
In \( \gamma \), the twins that form under sufficient stress are {1 1 1}<1 1 2>. The shear strain that this produces is large,\( 1 / \sqrt{2} \) . This is large even compared with transformation strains. This twinning tends to happen simultaneously with the glide of dislocations on the {111} \( \gamma \) planes.
A major advantage of TWIP steels is their f.c.c. austenitic structure. These steels do not exhibit a ductile to brittle transition, even at cryogenic temperatures, and remain ductile even to high strain rates, e.g., 103 s−1.
When plastically worked, twinning occurs in TWIP steels. This twinning increases the work hardening rate of these steels because the twinning boundaries act like grain boundaries, preventing the movement of dislocations. TWIP steels have high ultimate strengths, >1 GPa, and high formability.