Solidification of ternary alloys – Class I reactions
The ternary system below has a ternary eutectic invariant point. Associated with this point is a ternary eutectic (or class I) reaction:
\[ \rm{ L \rightleftharpoons \alpha + \beta + \gamma} \]Each of the bounding binaries has a binary eutectic point. A eutectic valley runs from each binary eutectic point to the invariant point.
>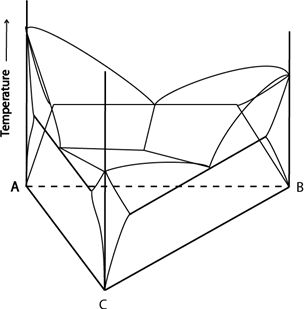
Figure 12: A ternary system with a ternary eutectic point. Adapted from https://commons.wikimedia.org/wiki/File:Space_diagram_of_a_three-component_system.jpg
This system has three single-phase regions (\( \rm{\alpha, \beta} \) and \( \rm{\gamma} \)), three two-phase solid regions (\( \rm{\alpha + \beta}, \rm{\alpha + \gamma}, \rm{\beta + \gamma} \)), one three-phase solid region (\( \rm{\alpha + \beta + \gamma} \)), three two-phase solid and liquid regions (\(\rm{ L + \alpha, L + \beta, L + \gamma} \)), three three-phase regions (\(\rm{ L + \alpha + \beta, L + \beta + \gamma, L + \alpha + \gamma} \)) and a single phase liquid region.
There are three kinds of solidification routes worth considering in this system:
- Alloys that do not pass through a three-phase region on cooling
- Alloys that pass through a three-phase region but not the invariant point on cooling
- Alloys that pass through the invariant point
The kind of solidification route an alloy falls into can be determined by observing which phase field its composition lies in at the invariant temperature.
An example isothermal section from a system with a class I reaction at the invariant temperature is shown below:
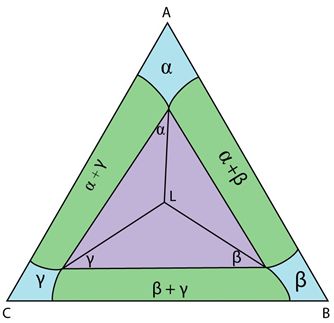
Figure 13: The invariant reaction plane for a ternary system with a class I reaction.
This is the invariant reaction plane. It is analogous to the invariant reaction line seen in some binary systems. This section shows four phases in equilibrium. This is shown by a tie triangle (\(\rm{\alpha + \beta + \gamma} \)) with the composition of the liquid plotted at the invariant point inside the triangle.
The position of the liquid within the tie triangle linking the other three phases is characteristic of class I reactions. The relationship of the liquid composition to the tie triangle linking the other three phases is different for each class of reaction and can be used to identify reaction types.
Any point within the tie triangle can be described in terms of proportions of the phases at the vertices. The liquid composition can be described as proportions of each solid phase. If the liquid composition lay outside the triangle this would not be possible.
For the liquid to decompose into the three solid phases, its composition must lie in the tie triangle for matter to be conserved. If the liquid lay outside the tie triangle, for example if it was poorer in C and lay closer to the AB join, then all three solid phases would be richer in C than the liquid. There would simply not be enough C in the liquid to form the three solid phases. The eutectic reaction could not occur.
At the invariant temperature, maximum solubility of unlike atoms in single phase solids occurs. If an alloy plots in a single-phase field at this temperature, it will cool without passing through a three-phase region. This cooling is analogous to an alloy cooling through a two-phase solid + liquid region in a system with complete solid solubility. This case has been discussed on previous pages in more detail.
If an alloy plots in a four-phase region at this temperature, it will undergo a binary eutectic reaction and pass through the corresponding three-phase region. This alloy will not cool through the invariant point as the system is completely solid at the invariant temperature and the invariant reaction requires a liquid phase.
The solidification of this kind of alloy is therefore analogous to the case discussed on the previous page of a system with a single eutectic valley. The alloy will cool through the single-phase liquid field into the two-phase field where a primary solid will form. The alloy then cools into and through the three-phase region with the proportions of the phase present changing (liquid decreasing while the two solid phases increase) as the tie triangle sweeps past the bulk composition. Once the tie triangle is such that the bulk composition lies on the side joining the two solid phases, the system is fully solid and the alloy cools through the two-phase solid region. This is discussed in more detail on the previous page.
If an alloy plots in the three-phase region at the invariant temperature, it undergoes the invariant reaction.
Alloy P in the diagram below undergoes the ternary eutectic reaction:
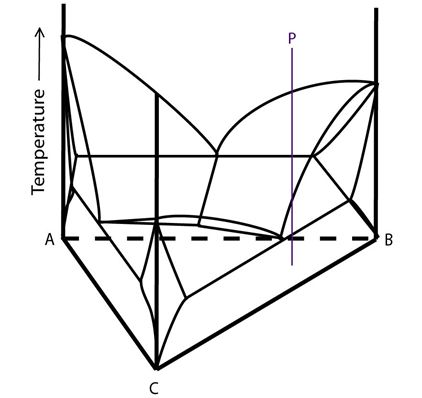
Figure 14: Composition of alloy P plotted onto a ternary system with a class I reaction. Adapted from https://commons.wikimedia.org/wiki/File:Space_diagram_of_a_three-component_system.jpg
The solidification of alloys undergoing the ternary eutectic reaction is more complex than that of other alloys in this system:

