Solidification of ternary alloys – one eutectic valley
The ternary system below has one eutectic valley. Two of its bounding binaries have a eutectic point and the third shows complete solid solution.
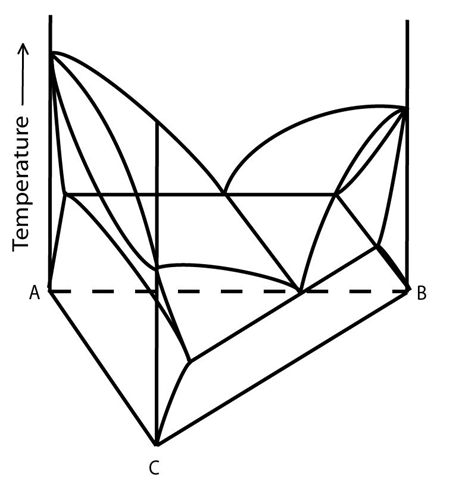
Figure 9: Space model of a ternary system with one eutectic valley. Adapted from https://commons.wikimedia.org/wiki/File:Space_diagram_of_a_three-component_system.jpg
This system has a single-phase liquid region (at high temperatures); two solid single-phase fields (\( \beta \) in the B rich corner and \( \rm{\alpha} \) which spans the AC join); two 'liquid + solid' two-phase regions \( \rm{(L + \alpha} \) and \( \rm{L +\beta} \)); a two-phase solid region \( \rm{\alpha +\beta} \)) and a three-phase field (\( \rm{L +\alpha + \beta} \)).
There are two solidification routes worth considering in this system:
- Alloys that do not pass through the three-phase region on cooling
- Alloys that do pass through the three-phase region on cooling
Solidification of Alloys that Do Not Pass Through the Three-Phase Region
Alloys whose compositions lie within the limit of maximum solubility of B in \( \rm{ \alpha} \) or A/C in \( \rm{\beta} \) do not experience the eutectic reaction. They do not pass through the three-phase region.
Alloys X and Y in the diagram below are both alloys that do not pass through the three-phase region.
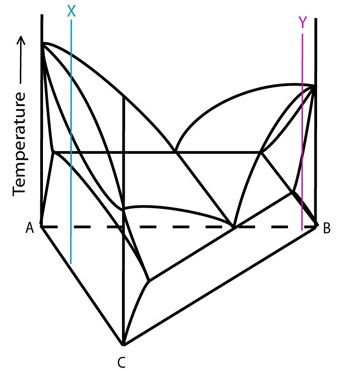
Figure 10: Compositions of alloys X and Y plotted onto a ternary system with a single eutectic valley. Adapted from https://commons.wikimedia.org/wiki/File:Space_diagram_of_a_three-component_system.jpg
As Alloy X cools from the liquid phase field, it passes across the liquidus into the \( \rm{L + \alpha} \) region. At this point, solid \( \rm{\alpha} \) begins to form. In a similar way to the complete solid solubility case, the alloy cools through the two-phase region with the composition of the solid and liquid varying according to the ends of rotating tie lines. The proportion of the liquid in the system decreases as the alloy cools through this region until there is no liquid remaining. At this point, the alloy crosses the solidus into the single phase \( \rm{\alpha} \) region. The entire system is solid and single phase.
The solidification route for alloy Y is very similar to that for alloy X except that this alloy lies in the \( \rm{L + \beta} \) phase field. The alloys cools across the liquidus from the liquid phase field into the \( \rm{ L + \beta} \) two-phase field. The solidification of \( \rm{ \beta} \) occurs. As the alloy cools through the two-phase region, the compositions of the solid and liquid change and the proportion of remaining liquid decreases. The liquid is exhausted as the alloy crosses the solidus into the single phase \( \rm{ \beta} \) region. At low temperatures the alloy crosses into the two-phase \( \rm{ \alpha + \beta} \) region. If the alloy is cooled slowly enough, \( \rm{ \alpha} \) will precipitate at triple points and grain boundaries until equilibrium proportions of each phase are obtained.
Solidification of Alloys that Do Pass Through the Three-Phase Region
Any alloy that passes through the three-phase region experiences the eutectic reaction. These alloys lie outside the limits of maximum solubility of B in \( \rm{ \alpha} \) or A/C in \( \rm{ \beta} \).
Alloy Z in the diagram below is an alloy that passes through the three-phase region.
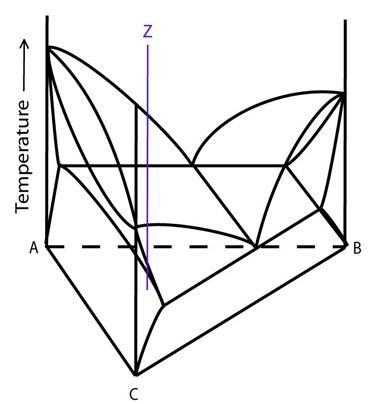
Figure 11: Composition of alloy Z plotted on a ternary system with a single eutectic valley. Adapted from https://commons.wikimedia.org/wiki/File:Space_diagram_of_a_three-component_system.jpg
The solidification of alloys passing through the three-phase region is more complex than that of alloys that do not:

