Solidification of ternary alloys – complete solid solution
The ternary system below shows complete solid solubility. Each of its bounding binaries show complete solid solubility.
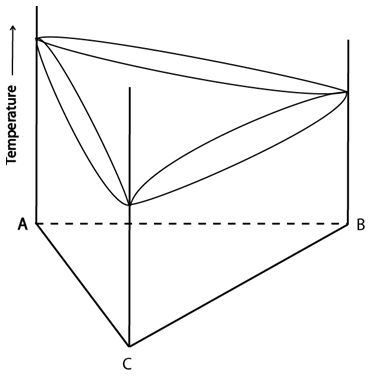
Figure 7: Space model for a ternary system with complete solid solubility. Adapted from https://commons.wikimedia.org/wiki/File:Space_diagram_of_a_three-component_system.jpg
This system has a single-phase liquid region (at high temperatures); a single-phase solid region (at low temperatures, spanning the whole composition triangle) and a two-phase (liquid + solid) region.
All alloys in this system cool through all three regions.
Initially they cool through the liquid phase field. When they reach the liquidus, alloys enter the two-phase field where the solid phase begins to form and the proportion of liquid in the system decreases. Once all the liquid has been exhausted, the alloy passes across the solidus into the single-phase solid field.
When in the single-phase fields (the liquid at high temperatures and the solid at low temperatures), the alloy is homogenous, and its composition is the bulk composition.
When the alloy is in the two-phase field, it is heterogenous. The compositions of the solid and liquid at each temperature are given by tie lines which span the two-phase region. These tie lines change with decreasing temperature so the equilibrium compositions of the solid and liquid also change with decreasing temperature.
Consider alloy W in the diagram below:
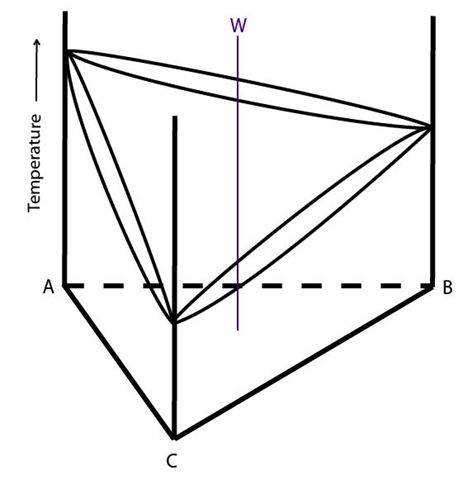
Figure 8: The composition of alloy W is plotted in a ternary system showing complete solid solubility. Adapted from https://commons.wikimedia.org/wiki/File:Space_diagram_of_a_three-component_system.jpg
The solidification behaviour of alloy W is described below:

