Questions
Quick questions
You should be able to answer these questions without too much difficulty after studying this TLP. If not, then you should go through it again!
-
In a ternary system ABC at a specific temperature there is a three-phase equilibrium of α, β and γ such that the compositions of the phases (by weight) are:
α 75% A 15% B 10% C
β 20% A 50% B 30% C
γ 5% A 30% B 65% C
An alloy lies in this three-phase region. It consists of equal proportions of α, β and γ. What is its composition? -
A two-phase region exists in a ternary system. Which set (or sets – you may choose more than one) of variables can be used to define the system?
Deeper questions
The following questions require some thought and reaching the answer may require you to think beyond the contents of this TLP.
-
What is the composition of alloy X in the diagram below?
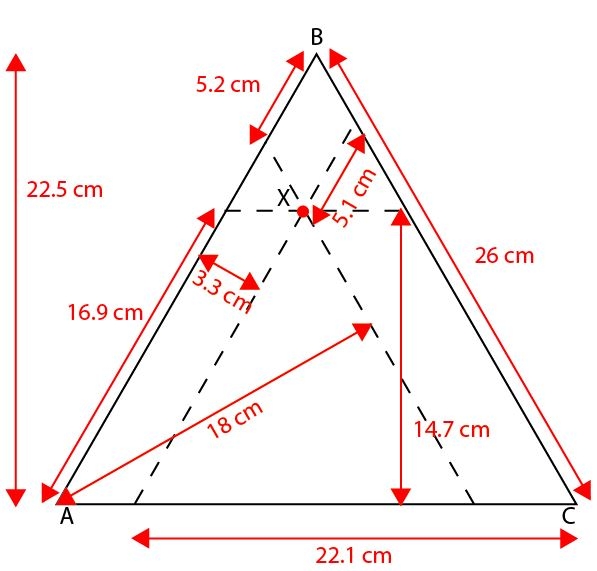
(a) 70% A 20% B 10% C
(b) 15% A 75% B 10% C
(c) 40% A 55% B 20% C
(d) 20% A 65% B 15% C -
Which of the following statements about vertical sections is incorrect?
-
Which of the following diagrams has possible tie lines shown?
-
In the diagram below, which two phase fields are separated by the shaded surface?
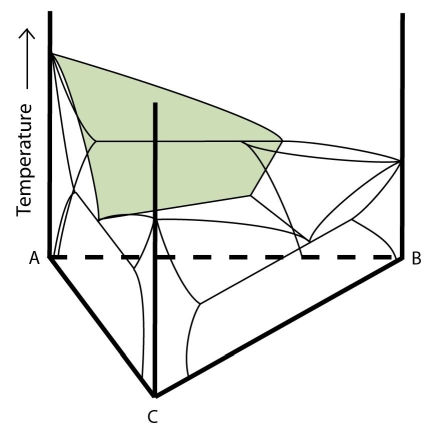
-
During a univariant eutectic reaction (where a binary eutectic reaction is extended into the ternary space, shown by a eutectic valley) the proportions of phases present change as the reaction proceeds (the proportion of L decreases to zero and the proportions of the two solid phases increase). Why is this?
-
Which of the following is associated with a class II reaction?
-
Consider a ternary phase diagram where one of the bounding binaries has a eutectic point and the other two show complete solid solubility. The diagram below shows such a system (the ternary space is left intentionally blank):
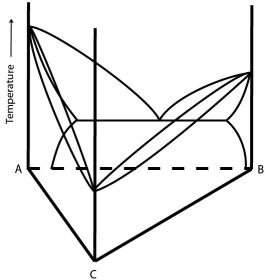
Sketch what an isothermal section for this system might look like a) above the eutectic temperature but below the melting point of B b) below the eutectic temperature and c) below the melting point of C.

