Introduction
Phase diagrams provide thermodynamic information for a system – the equilibrium phase or phases for a given set of conditions (usually temperature, pressure, composition). From phase diagrams, the proportions and compositions of the phase or phases present can also be inferred. This information is incredibly valuable when considering the behaviour of a system during heating and cooling.
Cooling and heating regimes (initial cooling rate, heat treatments, the reactions the alloy experiences or bypasses, etc.) determine the microstructure of an alloy, which in turn strongly influences its mechanical properties. Phase diagrams are a vital tool in optimising the microstructure of an alloy for a given purpose.
Many industrially important systems can be sufficiently described by binary phase diagrams but as alloys become more complex and incorporate more components, more complex analysis is needed.
Ternary phase diagrams are used to analyse ternary systems (alloys composed of three components). They can also be used in the simplified analysis of higher order systems (quaternary, quinary, etc).
The complexity of phase diagrams increases with the number of components involved (for example binary systems being more complex than unary systems) so the creation, analysis and interpretation of ternary phase diagrams is more involved than it is for binary phase diagrams.
A typical, simple ternary phase diagram is shown below:
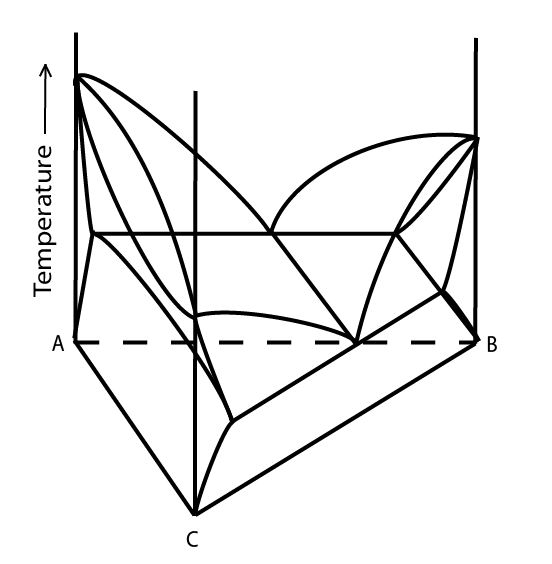
Figure 1: A relatively simple ternary phase diagram showing a single eutectic valley. Adapted from https://commons.wikimedia.org/wiki/File:Space_diagram_of_a_three-component_system.jpg
Most real ternary systems are very complex and so simpler, schematic ternary systems are used throughout this TLP to convey key points. These schematic systems have generic components (A, B and C) and up to three solid phases (\( \alpha \) - an A-rich phase, \( \beta \) - a B-rich phase and \( \gamma \) - a C-rich phase). The liquid phase is denoted by L in all schematic systems.
While very powerful tools, ternary phase diagrams suffer the limitations of all phase diagrams. They show only thermodynamic information with no consideration for kinetics or non-equilibrium systems.

