Tie lines and tie triangles
Tie Lines
Tie lines are isothermal and isobaric lines which span two phase regions.
Each end of a tie line represents the composition of a phase. Any point along the line can be described as a proportion of each of the endmember phases.
In binary systems, tie lines are horizontal lines spanning the area that represents the two-phase region. Each tie line is associated with a specific temperature, and they run from one bounding curve of the two-phase region to the other bounding curve.
In ternary systems, tie lines are horizontal lines (they lie in the planes of isothermal sections) and run from one bounding curve of the two-phase region to the other bounding curve. There are multiple tie lines for a given temperature.
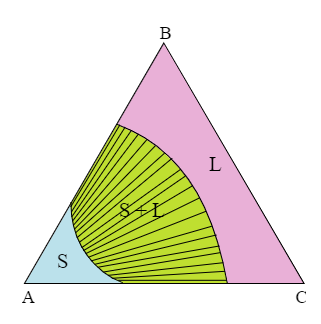
Figure 4: Isothermal section from a system with complete solid solubility. The two-phase region has tie lines plotted.
The figure above is of an isothermal section through a ternary phase diagram showing complete solid solubility. This isothermal section has the tie lines for the two-phase region plotted onto it. The whole section is taken for a single temperature but there are many tie lines.
The positions of tie lines on an isothermal section cannot be inferred from the section alone. Often, tie lines are plotted onto isothermal sections but if they are not then the tie lines cannot be guessed.
Restrictions on Tie Lines in Ternary Systems
Two tie lines at a given temperature can never cross.
Consider an isothermal section of a two-phase region in ternary space which has two tie lines that cross. Consider the alloy with the composition of this crossing point. When this alloy reaches the temperature of the crossing tie lines there is no deterministic outcome.
If the Gibbs free energy associated with each pair of phases (a tie line defines each pair) is the same, then the system would decompose into four phases. This violates the Gibbs phase rule and is impossible.
As the Gibbs free energy associated with each pair of phases cannot be the same, it must be lower for one pair (for one tie line) than the other. The system will then decompose into two phases given by the tie line with lower Gibbs free energy. This makes one of the tie lines redundant.
Crossing tie lines, therefore, cannot exist at a given temperature.
It follows then, that if a two-phase region spans from one binary to another binary (as it does in the figure above) then the tie lines fan out between the two binaries. This allows the tie lines at the edge of the ternary space to be parallel to their respective binaries and for no tie lines to overlap. The tie lines rotate smoothly from being parallel to one binary at one edge of the two-phase region to being parallel to the other binary at the other edge of the region.
Moving down temperature, tie lines rotate in the direction of decreasing melting point of the endmember components (For example, in the above system A has the highest melting point, then B, then C. As temperature decreases the tie lines will rotate from A to B and then round to C). This is known as Konovalov’s Rule.
The rotation of tie lines limits the use of vertical sections. It is unlikely that the alloy of interest lies on a tie line that is also in the plane of vertical section at one temperature, let alone a range of temperatures. In nearly every case the ends of the relevant tie line (which represent the compositions of the two phases) do not lie in the plane of the vertical sections. Subsequently, a horizontal (isothermal) line drawn joining the edges of a two-phase region is not a tie line; it does not link the compositions of the two phases that form.
There are cases in which vertical sections can be treated like binary phase diagrams. If a congruently melting compound occurs in one of the binaries making up the ternary phase diagram, then a section from the third component to this compound can be used to read off compositions of phases. These sections are often known as pseudo binary sections.
There is an example of a pseudobinary in the Au-Pb-Sn system. AuSn is an intermetallic phase which melts congruently. The vertical section taken from the Pb corner to this compound is a true binary. Isothermal lines on this diagram are tie lines. This vertical section is shown below:
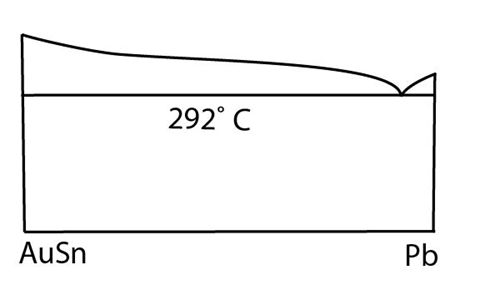
Figure 5: AuSn to Pb phase diagram - a pseudo binary from the Au-Sn-Pb system. Adapted from figure 1. https://www.sciencedirect.com/science/article/pii/002250886790094X
Tie Triangles
Tie triangles are isothermal, isobaric triangles which span three phase regions.Each corner of a tie triangle represents the composition of a phase. Any point within the triangle can be described in terms of proportions of these phases - in a similar way that any point within the composition triangle can be described in terms of proportions of each component, though tie triangles are rarely equilateral.
For tie lines, the proportions of each phase could be determined by simply using the lever rule. The process for determining the proportion of each phase in a three-phase region is slightly more involved but quite similar to determining the proportions of each component in a ternary alloy.
Similar to finding the proportions of two components in an alloy, finding the proportions of two phases allows the third to be calculated without doing a construction on the tie triangle (as the percentages of the three phases must sum to 100%). However, it is good practice to do a construction for all three phases and check that they sum to 100% to ensure that no mistakes have been made.
Unlike tie lines in ternary systems, each three-phase equilibrium only has one tie triangle at any given temperature.
On an isothermal section, a three-phase region is represented by a tie triangle which is associated with three two-phase regions generally along the edges of the triangle) and three single phase regions (at each vertex of the triangle).
Usually, the equilibrium composition of the phases involved in a tie triangle vary with temperature. Subsequently, when comparing isothermal sections for multiple temperatures, tie triangles will appear to move across the composition triangle.
Three phase regions in ternary systems are associated with a three-phase reaction (eutectic or peritectic for example) in one of their binary systems. The binary reaction is invariant (occurs at a specific temperature and composition), but the three-phase region is univariant (exists over a range of temperatures and compositions - see Applying the Gibbs Phase Rule to Ternary Systems).

